
Below is a list of 1248 of all serious adverse events listed as well as total adverse events of 9845 and 332 death reported from the 15th of December 2020 to 22nd of January 2021. It is a very sobering read that is heart breaking.
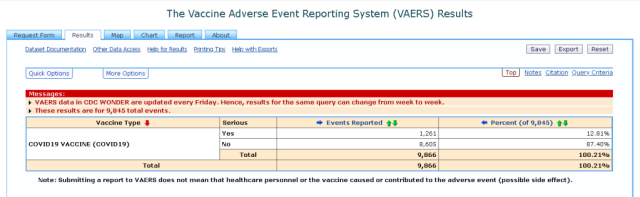

Source: The Vaccine Adverse Event Reporting System (VAERS) Request
| COVID19 VACCINE (COVID19) | “rPfizer-BionNTech COVID-19 Vaccine EUA 5-7 minutes after the vaccine Associate stated she did not feel right, mentioned chest pain. “”My chest feels funny. It feels like when you have really bad heartburn coming on””. “”I feel flushed like when you get contrast for a CT””. Pulse 90 BP 160/90 checked later 130/90″ | |
|---|---|---|
| COVID19 VACCINE (COVID19) | Anaphylaxis; throat closing; tongue swelling; Peripheral shutdown; This is a spontaneous report from a contactable physician and pharmacist received from the Agency. The regulatory authority report number is GB-MHRA-WEBCOVID-20201209083957 and ADR 24541453-001 and ADR 24541453-002. A 49-year-old female patient (healthcare worker) received BNT162b2 vaccine (Batch/lot: EJ0553) on 08Dec2020, via an unspecified route of administration route at single dose for COVID-19 immunization.The patient had a pertinent medical history of food allergy (Lemon/lime, egg and meringue cheesecake) with no known previous reaction to vaccines. Concomitant medications included progestogen orally and an unspecified medication topically, both for menopause and Vitamin B12 orally for vitamin B12 deficiency. On 08Dec2020 during post-vaccination observation the patient developed within minutes throat closing, tongue swelling, peripheral shutdown, no wheeze, chest clear. These events were reported as anaphylaxis, and required hospitalization. Vaccinated at hospital and received vaccine as she is patient facing employee. Otherwise fit and healthy, no cardiovascular, respiratory, gastrointestinal or neurological disease. No history of allergy to medicines. History of a similar allergic reaction to lemon/lime and meringue cheesecake. After 3 mouthfuls of cheesecake, onset of reaction. Required adrenaline, ambulance and treatment as inpatient. Allergy blood tests and skin tests inconclusive (unknown what tested for). Carries Epi Pen but never used. Has remained on a gluten and dairy free diet since the reaction. On the day of vaccination, her presenting anxiety was possible allergy to eggs. Within approximately 8 minutes of vaccination, she started to cough and became hypertensive (peak 175mmHg systolic), with a heart rate (HR) of 110 beats per minute (bpm) – pulse oximetry, no trace. No wheeze, no erythema of oral mucosa, no swelling. Started clawing at her neck and described feeling of itching internally. It was reported the patient carried an adrenalin autoinjector (EPIPEN). The patient was treated with IM adrenaline, IM chlorphenamine maleate (PIRITON), IM hydrocortisone given with minimal improvement and given two nebulised adrenaline (adrenaline nebulizers) which resulted in rapid resolution of symptoms (15 minutes elapsed between administration of IM adrenaline and nebulised adrenaline). Around 20 minutes later her symptoms returned. Given nebulised adrenaline with rapid recovery. Admitted to short stay unit (emergency department (ED)) for observation and discharged around 19:30 on 08Dec2020. No tryptase testing performed, no other blood tests. There was no reaction at the injection site. On 09Dec2020 the patient was at home and reported feeling tired, with heavy limbs. She is apyrexial with no ongoing signs of allergy. Lab tests on 08Dec2020 includes: Blood pressure systolic: 175mmHg, Heart rate: 110bpm and Pulse oximetry: No trace. The patient had recovered from the events in Dec2020. The events were considered serious medically significant, for hospitalization and for being life threatening. The patient has not had symptoms associated with COVID-19. Patient has not been tested or has had an inconclusive test for COVID-19 (as reported). Patient is not enrolled in clinical trial. The vaccine was given by hospital staff member. Follow up (10Dec2020): New information received from GB-MHRA-WEBCOVID-20201209083957 and ADR 24541453-002 includes: patient history, concomitant medications, lab tests, clinical course and the only event reported was anaphylaxis.; Sender’s Comments: The reported information is limited. Based on the close temporal relationship, the subject’s signs and symptoms, being, at least in part, suggestive of anaphylaxis and the past medical history of allergy, there is a reasonable possibility that the events are related to BNT162 vaccine. | |
| COVID19 VACCINE (COVID19) | HYPOTENSION; Supraventricular tachycardia (SVT); CHEST PAIN; DIZZINESS; NECK TIGHTNESS; ERYTHEMATOUS RASH; LOCALISED ITCHING; This is a spontaneous report from a contactable pharmacist and from the Regulatory Agency. The regulatory authority report number is GB-MHRA-WEBCOVID-20201209123237. A 54-year-old female patient with a medical history of supraventricular tachycardia (SVT) who has been symptom free for one year with no treatment after four episodes (date of diagnosis not provided) with no reported concomitant medications who received BNT162B2 (Reported as COVID-19 MRNA VACCINE BIONTECH; Lot number EJ0553) intramuscular on 09Dec2020 at 30 ug for COVID-19 vaccination. The patient experienced hypotension and supraventricular tachycardia (SVT) on the day of vaccination , at 12:00, associated with chest pain, dizziness, neck tightness, erythematous rash and localised itching. All the events were considered life-threatening. Clinical course was as follows: On 09Dec2020,he patient was noted as fit and well, prior to the vaccination. The first dose of vaccine was given at 10:37. At 12:00, the patient developed a sudden onset of SVT. The patient was visited by anaesthetist, monitored with a crash trolley and given oxygen. The patient’s heart rate was found variable at 180 to 230 beats per minute (BPM) and non-responsive to vagal manoeuvres/carotid sinus massage. Chest pain, dizziness and hypotension was associated with a heart rate greater than 200 BPM . Non-specific erythematous rash was visible on the neck with no evidence of angioedema, tongue swelling, mouth swelling or bronchospasm. The patient started complaining of neck itchiness and tightness awaiting for the ambulance. The patient was given chlorpheniramine, hydrocortisone and normal saline (1000 ml). Adrenaline and adenosin were withheld. The patient was in SVT with intermittent chest pain when she entered the ambulance. The medicines administered in the hospital hub setting were in line with the national vaccination program. The patient had not experienced symptoms associated with COVID-19. The patient had not been tested or has had an inconclusive test for COVID-19 (as reported). The patient was not enrolled in a clinical trial. The clinical outcome of SVT was reported as recovering. The clinical outcome of hypotension, chest pain, dizziness, neck tightness, erythematous rash and localised itching was unknown.; Sender’s Comments: The events of supraventricular tachycardia, chest pain, hypotension, dizziness, muscle tightness, rash erythematous and pruritus are unlisted in the BNT162 Investigator’s Brochure. The reported information is limited (e.g. lack of full cardiologic workup, blood testing etc.). Based on the close temporal relationship between vaccination and onset of the events, there is a reasonable possibility that the events are related to BNT162 vaccine in a subject with a medical history of SVT. | |
| COVID19 VACCINE (COVID19) | Swelling of hands follwed by angioedema | |
| COVID19 VACCINE (COVID19) | About 5 minutes after the vaccine developed chest tightness, increased work of breathing, palpitations and severe dizzyness. Transferred to the ED where i received oxygen, IV benadryl, IV fluids and monitoring. Released after about 4 hours and continue to take benadryl 50 mg PO q 4 hours. Also developed red facial rash (unknown time) Pain at injection site began the morning after the injection. | |
| COVID19 VACCINE (COVID19) | 40 year female received Pfizer-BioNTech COVID-19 Vaccine today Patient reported prior h/o severe allergic reaction to influenza vaccine with eggs preservative. She has received flu vaccine w/o egg w/o problem. Due to her prior history of severe allergic reaction/ anaphylaxis to another vaccine, in this case flu vaccine with eggs, we should proceed with caution. She was told we could defer vaccination until more information becomes available. She opted to proceed with receiving Pfizer-BioNTech COVID-19 Vaccine and be observed for 30 minute observation period. Patient developed throat tightening approximately 20 minutes after vaccination. She received EpiPen within 1 minute of symptoms and was sent to ER immediately in wheelchair by nursing staff. Patient was evaluated in ED and was hemodynamically stable. She was given IV benadryl and was stable throughout observation | |
| COVID19 VACCINE (COVID19) | Approximately 10 minutes after vaccine administration, patient reported wheezing and coughing. Patient received epinephrine IM, IV Benadryl, IV solumedrol and racemic epinephrine SVN. Patient never developed a rash, hypotension, swelling of the lips, mouth or tongue, other GI side effects . Per ER attending and admitting physician, this reaction seems to be a clear exacerbation of the patient’s tracheomalacia. The patient was more responsive to racemic epi SVN as opposed to IM epi. Patient admits that psychological stress may have been a component of her symptoms. The admitting physician does not consider this to be an anaphylactic reaction to the vaccination. | |
| COVID19 VACCINE (COVID19) | 40 min after injection my throat and tongue started to feel weird and tight, pharmacy at my work hospital gave me 25 mg Benadryl and 650mg Tylenol. At about 1 hr 45 min after injection my throat got to the point of so swollen and itchy I couldn?t swallow. I went to nearest emergency room hospital they administered decadron orally, Pepcid P.O., and Toradol via IM. | |
| COVID19 VACCINE (COVID19) | Seizure approximately 24 hours after vaccination | |
| COVID19 VACCINE (COVID19) | “5 minutes after the Pfizer Covid-19 vaccine administration, the patient developed flushing, hives, felt warm and eventually short of breath. She started to wheeze and was wheeled into ER c/o “”I can’t breathe while holding throat and thrashing with facial flushness noted. PT took 2 Benadryls and had several Epi shots. She was then discharged from the ER and later on that day, started to feel short of breath again. In the ED today she was audibly gasping for air, however had no wheezing, had a normal saturation and a normal blood pressure. She had taken another dose of her EpiPen IM and diphenhydramine 50 mg by mouth prior to coming. She was then admitted to the hospital for further observation. While on the floor, she started to feel short of breath again (about 9 am on 12/18/2020), which required an RRT . Patient received another dose of diphenhydramine IV, methylprednisolone 125 mg IV and several doses of IM epinephrine. She also required oxygen. She was then transferred to an ICU for further care.” | |
| COVID19 VACCINE (COVID19) | Not all or limited to: anaphylactic reaction: Feeling lump in throat, tongue feeling funny with numbness, feeling of hard to swallow, throat tightness, shortness of breath, tachycardia, tachypnea, pressure, tingling, and numbness from head to toe, dizziness/lightheartedness, cough, voice changes. | |
| COVID19 VACCINE (COVID19) | “Patient is a very pleasant 62 year old gentleman with a history of HTN, hyperlipidemia who presented with left facial numbness and left UE numbness. He states that it is worse medially on his arm. Present in upper and lower face. He states it started around 730 am this morning. He also notes intermittent “”foggy”” sensation since Monday associated with some blurred vision that comes and goes. Denies focal weakness, unsteady gait, difficulty with speech and swallow. He denies f/c, cp, sob, rash, pruritis, n/v/d, edema. He did receive the Pfizer COVID vaccine yesterday” | |
| COVID19 VACCINE (COVID19) | Angioedema | |
| COVID19 VACCINE (COVID19) | sob, nausea, malaise, vomit, chest pain, throat tightness | |
| COVID19 VACCINE (COVID19) | Resident became short of breath 7 hours after vaccine, went to hospital, was COVID+ | |
| COVID19 VACCINE (COVID19) | Day following vaccine, fever, sob – went to hospital, COVID+ | |
| COVID19 VACCINE (COVID19) | Presented with periumbilical pain to emergency department (patient works at Medical Center). Admitted to hospital for small bowel obstruction. Labs were consistent with dehydration (Hct 55, Cr 1.25), as well as CRP 1.10. A CT Abd/Pelvis identified proximal dilation and fecalization of small bowel, with a transition point in the left lower quadrant. Distal to the transition point, the small bowel appears thick, with hyperenhancement and inflammation progressing into the cecum. General Surgery was consulted and patient admitted to hospital for management of this small bowel obstruction. | |
| COVID19 VACCINE (COVID19) | rash appeared at site of injection, very painful, hot to touch, hard, with rash spreading to torso | |
| COVID19 VACCINE (COVID19) | 15 minutes after getting the vaccine began itching that quickly developed into rash/hives to face, neck, chest, abdomen. At 20 minutes post vaccine developed severe leg weakness with lightheadedness, chest tightness, and SOB. 22 minutes out collapsed to the floor unable to bear weight due to leg weakness and had severe cramping and tingling in legs, still unable to move them. Was rushed to the ER from employee health and arrived approximately 30 minutes post vaccine administration at that time there was significant mottling to arms and hands with polar nail beds. Vital signs were stable, no strider. Given Solumedrol, Benadryl, and Pepcid STAT. Rash/hives and SOB improved, but legs weakness/tingling, cramping did not and noted purple feet with cyanotic nail beds and mottling to hands/ arms that would come and go. Rash/hives reappeared much worse 2 horse post meds to face, neck, and upper chest. Was given another series of Solumedrol and Benadryl and admitted to the hospital. I am now 19 hours post vaccine with improved but persistent leg weakness, now able to bear my own weight independently and walk a few steps, but still having legs cramps and intermittent tingling to feet. Color has improved with resolved mottling/cyanosis. I continue to have hives reappear with scheduled Benadryl, Solumedrol, and Pepcid. | |
| COVID19 VACCINE (COVID19) | Chest Tightness and shortness of breath | |
| COVID19 VACCINE (COVID19) | Tingly sensations all over body on and off, like crawling feeling for 12 plus hours. No shortness of breathe noted or blisters/break outs. | |
| COVID19 VACCINE (COVID19) | Resident short of breath approximately 10 minutes post vaccination. Staff noted that his baseline upon activity and transferring is generally shortness of breath. Resident wears 2L NC, O2 saturation was 92%, increased to NC to 4 Liters O2 saturation up to 96%. 170/80, heart rate 94, regular rhythm. Sent to hospital. | |
| COVID19 VACCINE (COVID19) | Rach, tachycardia, palpitations, shortness of breath shortly after receiving vaccine – given epi, solumedrol, Benadryl. persistent symptoms the following day. | |
| COVID19 VACCINE (COVID19) | 12 minutes after injection, I felt flushed and dizzy. They hooked me up to a vital sign monitor which showed my heart increasing to 133 bpm, SaO2 98%. A manual blood pressure check was 168/110. My heart felt like it was pounding, I was hot and sweating. After 10 minutes or so, I felt increasingly dizzy and my vision started fading. VS still showed tachycardia and hypertension. It became difficult to swallow and my tongue was feeling fat. A Rapid Response Team was alerted, they started and IV, and took me to the Emergency Department. I became very cold and shaky. My hands and feet became a little mottled. They gave me 50 mg IV benedryl, 20 mg IV pepcid, a dose of solumedrol, and IM epinephrine 0.3mg, and 1 Liter of fluid. My symptoms resolved and I was discharged home a couple hours later. | |
| COVID19 VACCINE (COVID19) | Patient presented to the emergency department at 8:45pm on 12/18/20 with lower abdominal pain, nausea, vomiting, and constipation that started approximately 2 hours prior to presentation, at approximately 6:45pm. Her labs were significant for a lipase of > 6000 IU/L, and a CT scan of her abdomen/pelvis was done that demonstrated evidence of acute pancreatitis. Given the fact that she does not have a history of heavy alcohol use, with normal triglycerides and no evidence of gallstones on her current admission, and no recent gastroenterology procedures, there is no clear etiology of her pancreatitis; concern for post-vaccination pancreatitis. The patient is currently admitted to the hospital, on hospital day #1 of her current condition. | |
| COVID19 VACCINE (COVID19) | Angioedema, hives, tachycardia, shortness of breath | |
| COVID19 VACCINE (COVID19) | acute, mild pancreatitis, associated with symptoms associated with Nausea and vomiting and abdominal pain. Patient’s symptoms started 1 day after her vaccination. | |
| COVID19 VACCINE (COVID19) | Patient is a pleasant 83 y.o. female pediatrician with history of Sjogren’s, hypothyroidism, hyperlipidemia, hypertension who had been at Hospital to get her Covid vaccine. 30 minutes after doing so she reports being in the lobby and about to walk upstairs and feeling fine. The next thing she knows she wakes up on the stairs with her nose and face bleeding surrounded by healthcare team. She denies any precipitating symptoms such as chest pain, shortness of breath, fevers dizziness, headache. She reports feeling well otherwise in the last few days. I did a thorough bony palpation exam including spine and he only point of tenderness besides on her face was the area above her right ankle. She does not have a history of syncope or collapse | |
| COVID19 VACCINE (COVID19) | Patient had vaccine at 1330 on 12/20. At around 1815 she began experiencing heart palpitations. She presented to the ED and she was found to have a heart rate in the 130s. EKG showed junctional tachycardia. She was given 6mg of adenosine and an EKG was repeated and showed sinus tachycardia. Eventually her heart rate decreased to the 70s-90s. She was noted to have a potassium of 3.4 which was repleted. She was admitted overnight for observation. In the morning her potassium was normal and she remained in sinus rhythm. She was discharged later that afternoon. | |
| COVID19 VACCINE (COVID19) | The patient was well prior to vaccination (12/17). The day after, he felt mildly unwell and had a low grade fever. The following day, he had a fever of 102. He received 1L of fluid at Urgent Care and had a BP ion the 80s. Shortly thereafter, he felt palpitations and developed AF. He came to the hospital where he was tachycardia to 200 bpm and hypotensive to SBP70s. He received aggressive fluid resuscitation (4L), IV metoprolol and was started on empiric Abx. Within several hours, the HR lowered, BP increased, and AF spontaneously converted to sinus. He had no dysuria. Curtures so far have not shown growth at our hospital. Urinary culture from urgent care has reportedly shows 20k gram positive cocci. | |
| COVID19 VACCINE (COVID19) | 12/18/2020 morning I started to have runny nose, achy ‘like coming down with a cold’. Late evening and early morning 12/19/2020 tender lymphnodes, same side of vaccination. As of 12/20/2020 barely palpable anymore. Temperature was normal. I took ibuprofren and tylenol; 12/18-12/19. Congested, nasal drip ‘ 48 hours of feeling worn down and achy’. Flu shot in October 2020 | |
| COVID19 VACCINE (COVID19) | Ventricular tachycardia. Defibrillator paced me out of rhythm. I have had my ICD for 3 years. This is the first abnormal rhythm I have had where it delivered a therapy to abort it. | |
| COVID19 VACCINE (COVID19) | Throat closure (angioedema/anaphylaxis) requiring ambulance transport to Hospital emergency room and stay IV infusion of Benedryl, solumedrol, and Pepcid with excellent results. Observed twelve hours, then discharged. | |
| COVID19 VACCINE (COVID19) | Within a few minutes of taking the vaccine, my lower lip began swelling. I was moved to the emergency department of Hospital and monitored and treated for four hours. Then I was released. At around 1:30 p.m. I felt my skin singling and started having difficulty breathing. Since I was no longer at my work (Hospital) I went to the closest hospital. This reaction was much worse. My husband drove. My heart rate increased. I was released at around 6:30 pm | |
| COVID19 VACCINE (COVID19) | I had no reaction following the vaccination. The next day I had very mild soreness at the injection site. The next morning (about 36 hours after the vaccination) I woke up with fatigue and a sore throat. I had breakfast and about 10 minutes later I vomited everything (projectile vomiting, no nausea or abdominal pain). An hour later I had episode of severe watery diarrhea (just one episode). Felt very weak so I decided to sit down, stumbled to a chair, and then proceeded to have a syncopal episode with about 4 minutes of seizure like activity (witnessed, I don’t remember that part). Decided to go to the ER, where I had labs, EKG, CXR, head CT scan, MRI and EEG. I was admitted for 24 hour observation, all the tests were normal. | |
| COVID19 VACCINE (COVID19) | Day 1: palpitations, dizziness Day 2: headache, redness, swelling, itching on vaccination site; GI disturbance Day 3: vaccination arm had paresthesia, heaviness, almost stroke-like symptoms; the symptoms started suddenly ** treated in the ER; IV steroids- for possible allergic reaction; tpa- for the stroke-like signs; halfway through the tpa, I felt an improvement with the arm | |
| COVID19 VACCINE (COVID19) | Do no suspect that vaccine caused patient condition and resulting inpatient admission. Suspect patient had COVID-19 at time of vaccination, but had not developed symptoms yet. Here is timeline: Patient went to ED on 12-18-2020 at 22:51 with complaint for fever and shortness of breath. Patient ended up testing positive for COVID-19, 12-19-2020 00:09, but was not symptomatic at time of vaccine. As of 12/21/2020 12:04 pm, patient is still inpatient and on comfort care/hospice. | |
| COVID19 VACCINE (COVID19) | severe abdominal pain experience 2 days post vaccination of dose 1 of 2. Diagnoses with early acute appendicitis on Friday December 18th and had a laproscopic appendectomy on Saturday December 19th. | |
| COVID19 VACCINE (COVID19) | Patient received Pfizer COVID 19 vaccine last Thursday 12/17. Admitted today (12/21) with bleeding and low platelet count – working up for ITP, TTP. Given recency of vaccination and no known contributory allergy or medical history, physician thought potentially associated with vaccination. | |
| COVID19 VACCINE (COVID19) | Pt expressed feeling tachycardic, jittery, shaky, site edema, shortness of breath and dizziness. Pt received epipen 0.3 mg IM injection x1 dose and benadryl PO, responded favorably and transported to ED for follow up care. | |
| COVID19 VACCINE (COVID19) | Nurse reports that patient had no problems after receiving vaccination. Patient went home and EMS was called early the next morning and team administered vaccination was contacted physician that the associate works for stating the patient had a heart attack. | |
| COVID19 VACCINE (COVID19) | Left sided weakness of face, arm and left leg, onset 15 minutes after receiving vaccination Brought immediately to ED, subjective feeling of closing of throat. Given IM epinephrine 0.3mg x 1. Upon evaluation in the ED by tele-neurology consult, she received 88.5mg of alteplase on 12/20/20 at 1721 She was admitted to the Intensive Care Unit on 12/20/20 at 2208 Seen by neurology on 12/21/20 at 1227. Evaluation showed weakness on the left side but is noted that it could be effort-related. Neurologist noted that patient was treated with alteplase; CT angiogram showed no significant blockage or stenosis. Noted that this is could be related to a vasovagal effect, psychogenic or an acute ischemic stroke. As of 12/21/20 at 1615, attending provider noted left-sided paresthesia, left-sided tics and possible transient ischemic attack | |
| COVID19 VACCINE (COVID19) | Rapid heart beat, difficulty breathing, itching, rash, clamminess, cold sweats, nausea | |
| COVID19 VACCINE (COVID19) | waves of heat feeling; tachycardia; chest tightness; This is a spontaneous report from contactable physician via Pfizer Sales Representative. A 50-year-old female patient received bnt162b2, via an unspecified route of administration in 2020 at single dose for immunization. The patient’s medical history and concomitant medications were not reported. The patient experienced waves of heat feeling, tachycardia, chest tightness 15 min after receiving the vaccine in 2020. Patient received several doses of steroid and was kept overnight in hospital and recovered. The outcome of events was recovered in 2020. Information on the Lot/Batch number has been requested.; Sender’s Comments: The reported information is limited. Based on the close temporal relationship and the description of the events, waves of heat feeling, tachycardia, chest tightness, there is a reasonable possibility that the events are related to BNT162 vaccine.The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this reviewas well as any appropriate action in response, will be promptly notified to regulatory authorities, Ethics Committees, and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | Given the vaccine at 712 pm on 12/20/20. At approximately 715 pm, she began to clear her throat and then became unable to speak, followed by audible wheezes and short, shallow breaths. At 1923, Epinephrine was administered. At 1928, she was able to speak again and was transported to the ED. The patient reports after arrival to the ER, the symptoms returned. She was given PO Benadryl, followed by IV Benadryl, and then a 2nd dose of Epinephrine. She was admitted to the ICU for observation. | |
| COVID19 VACCINE (COVID19) | At work patient had ALOC x10 minutes. Rapid response called. Transf to Hospital (12/18-12/20). D/C Dx ACUTE CORONARY SYNDROME (NON-STEMI) | |
| COVID19 VACCINE (COVID19) | 57-year-old female history of hypertension, hyperlipidemia, type 2 diabetes, COPD, subsegmental PE is not on anticoagulation, multiple cardiac stents presenting with greater than 12 hrs of worsening left-sided chest pressure, headache and shortness of breath. Patient takes a daily aspirin and had no improvement of symptoms with her at-home nitroglycerin. Here afebrile, HTN, remaining vitals wnl. Non-toxic, in moderate distress 2/2 to pain. EKG with minimal ST depressions in leads II and III. Will plan for CXR and labs. Pt given zofran and morphine for pain control. Will give additional aspirin for total 324 mg in last 24 hrs. On re-evaluation, pt with mild improvement in pain. Troponin elevated at 0.18, remaining labs wnl. At this time concerned for NSTEMI, pt treated with 1 mg/kg of lovenox and MOD consulted for admission. MOD evaluated pt and cardiology was consulted. Given concerning PMHx and current hx of chest pain with findings consistent with NSTEMI, cardiology at recommended likely transfer for cardiac cath. Will pend repeat troponin and EKG for dispo decision. | |
| COVID19 VACCINE (COVID19) | Approximately 65 hours post-vaccination patient felt profound fatigue, no appetite, and had increase in baseline chronic cough, and anosmia. Patient was admitted to the hospital on 12/21 due to worsening respiratory symptoms that required supplemental oxygen-initially 2L via nasal cannula. Patient was upgraded to ICU-level of care at 6:30PM 12/21 to receive high-flow nasal cannula, and has had one episode of fever (100.6) 12/22 at 7:00 AM. | |
| COVID19 VACCINE (COVID19) | Burning in throat, flushed face and neck. Developed stridor. Benadryl 25 mg PO given, Epinephrine 0.5mg given IM, albuterol inhaler 4 puffs. Transferred to the Emergency department and admitted to the hospital. | |
| COVID19 VACCINE (COVID19) | Patient was administered the Covid19 vaccine. She was advised to wait 30 minutes post vaccination. While the patient was waiting, she reported having an itchy throat, throat tightness, then a hoarse voice and a cough developed. This happened at about 20 minutes after she received the vaccine. The patient was assessed by the nursing and provider staff. She received an adult epi pen injection and EMS was called. Patient was taken to the ER by EMS. She reports she received two more epi injections, benadryl, and Solu Medrol. She was stabilized. Patient was discharged from the ED after several hours. She then reports a second episode of throat tightening and worsening cough at 12:30 am and was taken by ambulance to the ICU and admitted. She is still in the hospital at this time 12/22/2020. | |
| COVID19 VACCINE (COVID19) | When I got the vaccination I was 32weeks pregnant and on Saturday I had spontaeous rupture of the amnotic fluids and went immediately to the hospital and was immediately given steroid, magnesium for the baby. And on Sunday around 3:45PM I got a second round of the steroids and was transferred for observation. On Monday, at 8:06am I went into early labor I delivered my baby at 33weeks gestation and she weighed 3lb 11oz. Expected Date of Delivery-2/8/2021. I was a high risk patient d/t Fibroids but have experienced no issues the entire pregnancy and my last ultrasound was 12/17 and baby was healthy with no complications at that time. | |
| COVID19 VACCINE (COVID19) | seizure, resident sent to er and in CCU | |
| COVID19 VACCINE (COVID19) | “bronchitis or pneumonia; pain down arm into fingers; injection site tingling; Chest felt heavy and difficulty breathing; Chest felt heavy and difficulty breathing; bronchitis or pneumonia; rash on both arms with bright purple skin; rash on both arms with bright purple skin; felt like everything was numb and like she was on drugs; felt like everything was numb and like she was on drugs; Severe headache; This is a spontaneous report from a contactable consumer (nurse) via a Pfizer sales representative. A female patient of an unspecified age received the first dose of the bnt162b2 (BNT162B2; also reported as COVID-19 VACCINE), via an unspecified route of administration on an unspecified date at a single dose for COVID-19 immunization. The patient’s medical history and concomitant medications were not reported. On an unspecified date, the patient experienced: bronchitis or pneumonia (medically significant), pain down arm into fingers, injection site tingling, chest felt heavy and difficulty breathing, rash on both arms with bright purple skin, felt like everything was numb and like she was on drugs, and severe headache; all of which required hospitalization. The clinical course was reported as follows: The female patient (nurse) took the first dose of the vaccine and experienced pain down arm into fingers, injection site tingling. The patient’s chest felt heavy and difficulty breathing; however, the “”tongue never swelled.”” Rapid response was called, and the patient was taken to the hospital. The patient was given a “”cocktail”” to treat the reaction. The patient “”felt like everything was numb and like she was on drugs”” The patient also felt like she had bronchitis or pneumonia. The patient also experienced a “”rash on both arms with bright purple skin””; along with a severe headache. Therapeutic measures were taken as a result of pain down arm into fingers, injection site tingling, and chest felt heavy and difficulty breathing. The clinical outcome of the events was unknown. No follow-up attempt possible; information about batch/lot number cannot be obtained.” | |
| COVID19 VACCINE (COVID19) | anaphylaxis | |
| COVID19 VACCINE (COVID19) | Received COVID-19 vaccine on 12/18/2020 around 1225pm, ten minutes later while being monitored I started to feel hot flash, got dizzy like about to pass out, I asked to let me lay down, I felt the medication bitter taste in the back of my throat, I was clammy pale, I lay on a stretcher and put my feet up elevated, rapid response was called and BP was checked and Spo2, my hands were getting cold and tingling I was talking to RN, another RN, after laying down for ten minutes I sit up I was getting my BP back to normal, I sat down in the chair again for another 10 minutes, I was offered to go to the ER but I decline, I said I was getting better, after 15 minutes I left monitored by my supervisor I felt the medication in my stomach, after the tingling my fingers were numbed for the next days until present. | |
| COVID19 VACCINE (COVID19) | HPI: 56 y.o. male with no pmhx c/o generalized bruising for 2 days, noticed small blood tinged spots generalized. Gradual onset, severe on severity, no alleviating or aggravating factors. Patient denies fevers, chills, N/V/D, abdominal pain. In ER: Platelet <1. Platelet transfusion in ER. Admitted for Thrombocytopenia/ITP | |
| COVID19 VACCINE (COVID19) | PT WAS OBSRVED IN HOLDING AREA LEANING FORWARD IN HER CHAIR ABOUT 7 MINUTES AFTER RECIEVING THE VACINE. RN ASSESSED AND NOTED: AUDIBLE WHEEZE, RESP 40/MIN, LIP SWELLING AND PT COMPLAINED OF NAUSEA. PT WAS ESCORTED TO ER IN WHEELCHAIR ACCOMPANIED BY 2 RN’S (2 MINUTE WALK) ONE HOUR LATER – AS REPORTED BY DR (ER) WORKING DIAGNOSIS – ANAPHYLAXIS / STATUS ASTHMATICUS MEDS RECIEVED: SOLUMEDROL 125, DIPHENHYDRAMINE 50MG, FAMOTIDINE 20MG –ALL IV EPINEPHERINE 0.3MG IM X1 FOLLOWED BY 0.3MG IV X 1 FOLLOWED BY 0.1MG IV X1 PT IS RECIEVING O2 – AND PROGRESSING TO BIPAP | |
| COVID19 VACCINE (COVID19) | Anaphylaxis/Angioedema Patient was given EpiPen 0.3 mg IM; Methylprednisolone 125 mg once; Diphenhydramine 25 mg IV push once; Famotidine 20 mg IV push once; Dexamethasone 10 mg IV push once Patient was intubated and put on propofol and midazolam drips for sedation | |
| COVID19 VACCINE (COVID19) | Received vaccine around 10:40 am, by 10:50 started to feel dizzy, eyes felt full, dry, tingly, swollen, voice became raspy and throat itched. Received 25 mg Benadryl PO at around 10:55. Face, arms, chest and abdomen developed a fine red itchy rash, tongue swollen and itchy, lips tingling, wheezing, blood pressure elevated, pulse thready given 25 mg PO Benadryl, taken to the Emergency Room, symptoms persisted, stomach hurt became nauseated, received IV solumedrol, Pepcid, IV fluids, nebulized albuterol. Sent home once stable after 3 hours, with instruction to take Benadryl every 4-6 hours fir the next 2 days, albuterol as needed, and prednisone for the next 5 days. | |
| COVID19 VACCINE (COVID19) | EMS called after patient displayed a heart rate of 160, a little over an hour after vaccine administration. Patient was taken to the hospital and diagnosed with an episode of RVR Afib; she was admitted to the hospital. | |
| COVID19 VACCINE (COVID19) | Patient experienced bronchospasm with coughing and tongue itching approximately 10 minutes after the injection. | |
| COVID19 VACCINE (COVID19) | patient felt slightly nauseated at 10 minutes after injection, then developed slight sweating; BP 160/81; 83 at 5:45 and then 158/87 with HR 82 at 5: 52 pm. Her lungs were clear, she was speaking in full sentences and was denying any chest pressure, her usual sense of asthma exacerbation. At 6:05 it was 164/83 with HR 79 and patient developed a dry cough; we decided to have her wait just a bit longer, then cough worsened, so at 6:25, decision was made to have patient seen in ER for further assessment, and en route in wheelchair to ER the dry cough became persistent, spasmodic and patient was unable to speak. Epi-Pen was injected in right mid thigh, and patient transported to ED urgent eval. She noted immediate palpitations, and slight improvement of breahting, was able to speak in four word sentences. On arrival to the ED, patient was administered Duonebs, Albuterol neb, IV Benedryl, IV Solumedrol; CXR was obtained, with results pending. Patient was sent to observation for ongoing monitoring and assessment of breathing. at 6:30 PM in the ER, she | |
| COVID19 VACCINE (COVID19) | Congenital anomaly or birth defect; This is a spontaneous report from a contactable consumer (patient). A 57-year-old female non-pregnant patient received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration on an unspecified date at single dose for covid-19 immunization. Medical history included high blood pressure and hypertension and more epileptic seizures. Concomitant medication included unspecified pneumococcal vaccine on 12Dec2020 for immunization. The patient previously received flu shot (Influenza virus vaccine) and pneumonia shot (Pneumonia vaccine) both on 20Oct2020 for immunization. The patient experienced congenital anomaly or birth defect (as reported) on an unspecified date with outcome of unknown. Information on the lot/batch number has been requested.; Sender’s Comments: The reported information is unclear. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to regulatory authorities, Ethics Committees, and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | Employee presented to COVID Clinic for Moderna COVID 19 vaccination 1st dose. Given to left arm. Left clinic prior to completing 15 minute observation time and told an MA in waiting area that she felt ill ot her stomach and having trouble taking deep breaths. Employee found in nearby Bathroom sitting on the floor, she had vomited, reported she was lightheaded, couldn’t breath, shaking, abdominal discomfort sweating, attempted to move employee to wheelchair, did respond well to transfer to Wheelchair, She reported symptoms worsening: HA, abdominal pain and developed blotchy skin, hyperventilating, and dizzy. CODE Blue called, patient given Epinephrine injection 0.5mg patient sent to ER | |
| COVID19 VACCINE (COVID19) | Onset of symptoms began approximately 1.5 hours after vaccination. Patient became weak c/o tongue swelling, nausea, difficulty breathing, numbness especially in lower extremities. Felt she couldn’t breath, numbness continued to get worse and affected her upper extremities as well, weak speech. | |
| COVID19 VACCINE (COVID19) | 48 y.o female with history of atrial tachycardia who presents to the ED via EMS for a possible adverse reaction to Covid vaccine. Patient received her Covid vaccine around 1600 today, and soon became diaphoretic, shaky, and lightheaded. She had presented to the emergency department. Currently she denies any chest pain, difficulty breathing, throat swelling, tongue swelling, or any other symptoms currently except for palpitations. áAllergic Reaction The primary symptoms are shortness of breath. The primary symptoms do not include cough, abdominal pain, vomiting, dizziness or rash. The current episode started 1 to 2 hours ago. The problem has not changed since onset. The onset of the reaction was associated with a new medication. Significant symptoms also include flushing. 48-year-old female with a history of atrial septal defect, status post atrial septal defect repair in 1980. She works as a nurse at Hospital and has been experiencing increasing rapid palpitations associated with chest pain, and hypertension. With her episodes, she experiences marked lightheadedness, dyspnea, and feeling marked anxiety, as well as chest tightness. She received the COVID-19 vaccine today and while waiting in the observation room,she started feeling unwell, with rapid palpitations, associated with lightheadedness and dyspnea. She last had a sustained episode 2 – 3 weeks ago and had presented to ER and Hospital. No syncope. No orthopnea, PND or increased lower extremity swelling. Active Hospital Problems á Diagnosis ? Atrial paroxysmal tachycardia ? History of repair of atrial septal defect á á á 1. Paroxysmal atrial tachycardia in setting of prior atrial septal defect repair – she is having breakthrough episodes through flecainide/digoxin – it is likely her atrial tachycardia is related to her ASD patch. Will hold flecainide/digoxin for now, and try to schedule an ablation during her hospital admission due to highly symptomatic episodes resulting in multiple ER visits. á 2. Acute renal insufficiency – most likely pre-renal – iv fluids started. á 3. Possible COVID-19 vaccine reaction – she probably had an incidental atrial tachycardia episode post vaccine administration, rather than an actual adverse reaction. Continue to monitor. | |
| COVID19 VACCINE (COVID19) | Seizure (Grand mal) | |
| COVID19 VACCINE (COVID19) | Acute allergic reaction; Unsteadiness; Confused; Dizziness; Exhaustion; Feeling drunk; This is a spontaneous report from a contactable physician manually downloaded from the database: GB-MHRA-WEBCOVID-20201211215403, Safety Report Unique Identifier GB-MHRA-ADR 24542614. An adult female patient received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration on 11Dec2020 at single dose for covid-19 immunisation. The patient medical history was reported without any specified term but with precise dates from 16Mar2020 to 28Mar2020, other history included depression, gastrooesophageal reflux disease and anxiety. Concomitant medication included influenza vaccine (INFLUENZA VIRUS) in Oct2020 for influenza immunisation, lofepramine hydrochloride for depression, omeprazole for gastrooesophageal reflux disease, propranolol for anxiety. The patient experienced unsteadiness, confused, dizziness, exhaustion, feeling drunk, acute allergic reaction on 11Dec2020. All events were reported as serious (medically significant, life threatening). Outcome of dizziness was recovered in Dec2020, outcome of exhaustion was not recovered, and outcome of other events were recovering. Information on the lot/batch number has been requested. | |
| COVID19 VACCINE (COVID19) | Diarrhoea; This is a spontaneous report from a contactable other healthcare professional via Agency and downloaded from the Regulatory Authority GB-MHRA-WEBCOVID-20201212222117, Safety Report Unique Identifier GB-MHRA-ADR 24542707 and EU-EC-10007191252. An elderly patient of an unspecified gender received bnt162b2 (batch/lot number not provided), via an unspecified route of administration in 2020 at single dose for COVID-19 immunisation. The patient’s medical history and concomitant medications were not reported. The patient experienced diarrhoea in 2020. The patient died due to diarrhoea on 10Dec2020. It was not reported if an autopsy was performed. No follow-up attempts are possible. Information on the lot/batch number not obtainable. No further information is expected.; Reported Cause(s) of Death: diarrhoea | |
| COVID19 VACCINE (COVID19) | 12-22 HPI 53-year-old female with a history of Addison’s disease, anaphylactic reaction who presents to the ED complaining of hives and shortness of breath. Patient reports that 3 days ago she received the COVID-19 pfizer vaccine. She reports that since that time she has developed progressively worsening hives on her legs and arms. Approximately 1 hour ago she began to develop shortness of breath and so she presented to the ER. Patient reports a previous history of anaphylactic reactions multiple times. Denies any other acute complaints at this time. MDM Patient came in with shortness of breath and hives. Suspect allergic reaction to the COVID-19 vaccine. Patient had already taken 50 mg of Benadryl. She was given Solu-Medrol and EpiPen. She reported feeling better with improvement in the pruritus. She reports that she has had rebound reaction requiring EpiPen at 24 hours. Given the distance that she lives from adequate medical care and the possibility for recurrent severe reactions, the patient will be hospitalized for further observation. 12-23 Female with history of asthma and addison’s had anaphylaxis to covid vaccine. Admitted over night to ensure that she did not rebound. Received IV Dex and this am has had no reoccurrence of hives or shortness of breath. Will discharge home on epipen, hydrocortisone prn, prednisone bid for 5 days. Return to ER or go to PCP for worsening symptoms. | |
| COVID19 VACCINE (COVID19) | Patient received covid 19 vaccine. She began to experience itching throat and swollen tongue. She was sent to the Emergency Department. She received IV Benadryl 50 mg, IV famotidine 20 mg and 125 mg IV solu-medrol. Around 930, patient stated that symptoms had resolved, except for tongue being slightly swollen. Patient was admitted to the observation unit of the hospital. | |
| COVID19 VACCINE (COVID19) | First night following after vaccine I woke up with chest pain ( i though pleuritic) which went away. I had mild body aches and fatigue, chills. next day I experienced chest( again I thought pleuritic ) discomfort especially when taking a deep breath. i felt better then had mild fatigue and body aches again. day 3 post vaccine I woke up with discomfort when taking a deep breath with continued discomfort. i felt tired through the day. Then that evening i developed SOB, severe palpitations and chest pain and went to ER. Diagnosis New onset rapid A fib. I was hospitalized and once my work up was finished and I had normal sinus rhythm I was discharged home the next evening. | |
| COVID19 VACCINE (COVID19) | Chest pressure, dizziness, increased troponin lab value. | |
| COVID19 VACCINE (COVID19) | ANAPHLACTIC REACTION, SOB, CHEST PRESSURE, TIGHTNESS IN THROAT, TACHYCARDIA | |
| COVID19 VACCINE (COVID19) | Initially started with nausea around min 5, shortly after then itching on arms. Around min 15 ?lump? sensation in throat. Around min 20 swelling of tongue, worsening feeling in throat, wheezing, itching around mouth. Sent to ER, received IM Epi, IV: Steroids, Benadryl, Zofran, Pepcid, Albuterol inhaler. | |
| COVID19 VACCINE (COVID19) | Asystole; Circulatory collapse; This is a spontaneous report from a contactable pharmacist received from Agency and downloaded from the Regulatory Authority-WEB GB-MHRA-WEBCOVID-20201214111558, Safety Report Unique Identifier GB-MHRA-ADR 24542972 and EU-EC-10007191566 received via Regulatory Authority. An adult female patient received bnt162b2 (batch/lot number not provided), via an unspecified route of administration on 13Dec2020 at single dose for COVID-19 vaccination. The patient’s medical history was not reported. Concomitant medication included sildenafil, acetylsalicylic acid, allopurinol, levothyroxine, spironolactone, amiloride hydrochloride, furosemide and desogestrel. The patient experienced asystole on 13Dec2020, circulatory collapse on 13Dec2020. The patient died due to asystole and circulatory collapse on 13Dec2020. It was not reported if an autopsy was performed. No follow-up attempts are possible. Information about batch number is not obtainable. No further information is expected.; Reported Cause(s) of Death: circulatory collapse; Asystole | |
| COVID19 VACCINE (COVID19) | Anaphylaxis; This is a spontaneous report from a contactable pharmacist. A 55-year-old female patient received the bnt162b2 (BNT162B2; also reported as: PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration on 17Dec2020 at a single dose for COVID-19 immunization. The patient’s medical history included eosinophil process allergic reaction, fish, iodine and shellfish allergy; all from an unknown date and unknown if ongoing. Concomitant medications were not reported. The patient previously took rabies vaccine for immunization and experienced anaphylactic reaction on an unspecified date. On 17Dec2020, the patient experienced anaphylaxis; which required hospitalization, and was assessed as medically significant. The patient was hospitalized for anaphylaxis from 18Dec2020 to an unknown date. The clinical course was reported as follows: The pharmacist called about a patient who received the COVID-19 vaccine on 17Dec2020 and started having a reaction approximately 30 minutes later. The patient used epinephrine (EPIPEN) and 50 mg of diphenhydramine hydrochloride (BENADRYL) and returned to the hospital on 18Dec2020. The patient was currently in the intensive care unit (ICU) receiving an epinephrine drip. The patient had a previous history of an anaphylactic reaction to the rabies vaccine, eosinophil process allergic reaction, fish, iodine and shellfish allergy. The patient was stabilized but continued to have reactions (not specified). The pharmacist had not seen the patient and was reaching out to Pfizer on behalf of the physicians. The pharmacist believed this had been reported by the hospital. The pharmacist had no patient information. Therapeutic measures were taken as a result of anaphylaxis. The clinical outcome of the event, anaphylaxis, was unknown. The batch/lot numbers for the vaccine, BNT162B2, were not provided and will be requested during follow up.; Sender’s Comments: Based on the information available, a possible contributory role of the suspect products cannot be excluded for the reported event anaphylaxis due to temporal association. However patient previous history of allergic reaction cannot be excluded to have played a contributory role | |
| COVID19 VACCINE (COVID19) | Vaccine administered at 0730. Pt went home and went to bed. Woke up at 0930 with shaking, chills and feeling like she was going to pass out. Evaluated in ER and noted to have sinus tachycardia in 140-160’s. Given 5mg IV Lopressor which took heart rate down to 110’s. Admitted to hospital 12/23/2020. | |
| COVID19 VACCINE (COVID19) | Left arm leg and face numbness bilateral legweakness Vaccine given at 915 am Symptoms started at 11 am Called Pfizer to report at 1151 am Saw primary doctor at 2 pm Return to primary md again 12/22/20 Referral to neurosurgeon 11/22/20 Hospitalized 11/22/20 to 11/24/20 lepto Meningeal inflammation hospital | |
| COVID19 VACCINE (COVID19) | Within minutes I had lower chest pain and wired sensation in my lungs. My throat was swelling. I was very hot and red. I the gurney my right arm was tingling. Opposite arm from vaccine. | |
| COVID19 VACCINE (COVID19) | throat swelling, SVT | |
| COVID19 VACCINE (COVID19) | 12/18/2020: COVID19 vaccine received. 12/19/2020: Patient noticed petechiae/bruising on arms, legs and face. Worsened over next 48 hours. 12/21/2020: Patient had blood drawn (CMP, PT/INR, CBC) at lab. 12/22/2020: Labs resulted; CMP and PT/INR WNL (exceptions: SCr 1.24, TBil 1.7); CBC with platelet count of 1,000 resulting in patient admission to Hospital. At admission he received 80 mg of prednisone, 40 g of IV Ig and a unit of platelets. 12/23/2020: Continued hospitalization. Patient’s platelets improved to 20,000 and he received another 35g of IV Ig. 12/24/2020: Patient discharged with platelets of 38,000. | |
| COVID19 VACCINE (COVID19) | He was fine ate lunch, in a room with a patient, felt light headed and dizziness, passed out, he became unresponsive, he was hypotensive, he is now in the ER. | |
| COVID19 VACCINE (COVID19) | Patient vaccinated at Nursing home. Transferred to ER the following day when patient developed fever and altered mental status. Found to have acute kidney injury on chronic kidney disease, hyperkalemia. Required emergent hemodialysis for hyperkalemia with ECG findings of peaked T waves. | |
| COVID19 VACCINE (COVID19) | Patient is a 47 y.o. female who arrived by Car presented to the emergency department for Stroke symptoms. Patient awoke at 6:15 this morning, some difficulty seeing out of the right eye and also was stumbling towards the left and to table. Concerned about things not being right so brought to the emergency department. Patient feels her speaking and swallowing are okay. She did drink a bit of coffee earlier. She denies headache or significant vision problems presently. Continues to not feel normal on her left side. No history of stroke and parents or siblings. She does give personal history of an occipital migraine many years ago at which time she did not have a headache but had some vision troubles. Physical Exam Vitals signs and nursing note reviewed. Constitutional: General: She is not in acute distress. Appearance: She is not ill-appearing or diaphoretic. HENT: Head: Normocephalic and atraumatic. Right Ear: Tympanic membrane normal. Left Ear: Tympanic membrane normal. Nose: Nose normal. Mouth/Throat: Mouth: Mucous membranes are moist. Pharynx: No oropharyngeal exudate or posterior oropharyngeal erythema. Eyes: Conjunctiva/sclera: Conjunctivae normal. Pupils: Pupils are equal, round, and reactive to light. Comments: Patient displays absence of left lateral movement Neck: Musculoskeletal: Normal range of motion. No muscular tenderness. Cardiovascular: Rate and Rhythm: Normal rate and regular rhythm. Heart sounds: No murmur. Pulmonary: Effort: Pulmonary effort is normal. Breath sounds: Normal breath sounds. Abdominal: General: Bowel sounds are normal. There is no distension. Palpations: Abdomen is soft. Tenderness: There is no abdominal tenderness. Musculoskeletal: Right lower leg: No edema. Left lower leg: No edema. Lymphadenopathy: Cervical: No cervical adenopathy. Skin: Findings: No rash. Neurological: Mental Status: She is alert. Cranial Nerves: Cranial nerve deficit (left facial droop, dysarthria) present. Comments: Patient’s speech seems a bit slurred to me. Absence of ocular movements towards left noted as well as upward movements. Tongue is midline. Patient is unable to shrug the left shoulder or lift the left arm off the bed. Grip strength is 4 out of 5 on the left. Left leg strength is 3 out of 5. Extremity strength on right arm and leg is 5 out of 5. After consultation with a neurologist, the patient is being transferred from the ED. | |
| COVID19 VACCINE (COVID19) | This resident received the first dose of the Pfizer Covid vaccine at the Covid vaccine clinic at medical Facility. He had a rapid response, experienced low blood glucose and low heart rate. NP and medical director were in attendance. Heart rate remained low despite all measures. He was transferred to Hospital for bradycardia. | |
| COVID19 VACCINE (COVID19) | Starting that night 12/19/20 at approx 8pm I became nauseous with a 100.8f fever. I felt achy for the 2 days with the fever subsided. On Monday 12/21/20 I was at work (Firefighter) when I started to have palpitations. I was able to obtain a 12 lead EKG right away which showed a ventricular rhythm lasting for approx 5 min. After that I was having multiple PVC’s / Ventricular beats. I was taken to Hospital where I was admitted. Over night the rhythm subsided, all my labs were good with other test’s being done. I was discharged home. I have not had any other episodes since Monday. I was cleared by our Occ Health Doc who suggested I fill this out. | |
| COVID19 VACCINE (COVID19) | 15 min after receiving Covid 19 vaccine patient started to feel like her heart was racing / felt faint. Burning feeling in upper thigh and pelvic area. BP 180/100 HR 130. Rapid Response called / transported to ER. Admitted for 24 hr observation.. Solu -medrol, Benadryl and Ativan given in ER. Released home the next day. 72 hrs later patient states she has numbness and tingling in hands and feet. 12/24/2020 patient reports she is feeling better today / no symptoms noted. | |
| COVID19 VACCINE (COVID19) | Patient presented with signs and symptoms of sepsis, developing over 12 to 24 hours 6 days after vaccination. was hypotensive and confused (beyond baseline) | |
| COVID19 VACCINE (COVID19) | Reported sensation of tongue swelling during post-vaccination observation at 10 minutes. Epinephrine was refused and she was taken to ED for observation where she was given oral dose of Benadryl and Pepcid. Discharged with instructions to return PRN and follow up with PCP. Elevated BP noted. | |
| COVID19 VACCINE (COVID19) | on 12/24/2020 the resident was sleepy and stayed in bed most of the shift. He stated he was doing okay but requested pain medication for his legs at 250PM. At 255AM on 12/25/2020 the resident was observed in bed lying still, pale, eyes half open and foam coming from mouth and unresponsive. He was not breathing and with no pulse | |
| COVID19 VACCINE (COVID19) | Acute NSTEMI with symptom onset 4 days after vaccination | |
| COVID19 VACCINE (COVID19) | listed before | |
| COVID19 VACCINE (COVID19) | Approximately 2 minutes after injection, felt flushed and tingly. This subsided, but developed a cough. Felt fine enough to leave the vaccination area after being monitored for 15 minutes. Cough continued, and developed a scratchy throat that eventually led to swelling of the throat at approximately 30-35 mins post administration. Sought care in the ED, where I was tachycardic and hypertensive. Received IV Benadryl, steroids, and IV fluids. Discharged home, but symptoms returned around 2pm. Sought care in a different ED, where I remained hypertensive and tachycardic. Received additional IV fluids, IV Benadryl and steroids. Eventually was treated with IM epinephrine after my heart rate was decreased to about 100bpm with IV metoprolol. | |
| COVID19 VACCINE (COVID19) | At the time of the injection sharp pain across my back , then at about 5 mins after feelings of light headedness, progressing pain across my back, trouble feeling like I could get enough air in with breathing and dizziness and I tried to get to the floor to sit or lay down but passed out. Then the next event I recall was a sharp pain in my thigh(apparently administered Eli pen) . I regained consciousness and was gasping andI was told I had been given a shot of epi. | |
| COVID19 VACCINE (COVID19) | Rapid onset of hoarseness, throat tingling and tightness | |
| COVID19 VACCINE (COVID19) | near syncope, hypotension, nausea/vomiting, tachycardia (120-150) within 5 minutes of administration. did not resolve and worsened within 1 hour. Pt went to ER for workups. Received IV benadryl without improvement. Admitted to hospital overnight for continuous cardiac monitoring. Improved overnight and discharged in the afternoon 12/24/20. | |
| COVID19 VACCINE (COVID19) | O had the vaccine at 9 am this morning waited 15 mins after vaccine before leaving while driving I had a pounding heart rate and hot I rolled down the window felt better. 1 hour later while at home.e started with nausea diarrhea rapid heart rate headed to medical office while in care tongue swelled I called 911 pulled over when the ambulance got to me my throat swelled and I had hives on chest they took me emergency while there I had sever pounding heart and vomiting treated with meds sent home with medication and benadryl | |
| COVID19 VACCINE (COVID19) | The patient developed palpitations, lightheadedness and nausea and came to the ED and was found to have sinus tachycardia. Unclear if it is a vaccine reaction or due to anxiety or severe iron deficiency anemia. | |
| COVID19 VACCINE (COVID19) | Dizziness, dyspnea, neck swelling | |
| COVID19 VACCINE (COVID19) | The patient was vaccinated on 12/17/20. Wife was diagnosis with COVID-19 on 12/18/20. He was diagnosis with COVID-19 on 12/21/20. Symptoms woresen on 12/26/20. And he had chest exam (x-ray’s), pneumonia bi-lateral and he was hostipalized on 12/26/20. | |
| COVID19 VACCINE (COVID19) | Fever, muscle aches, hypertension, rapid heart heart | |
| COVID19 VACCINE (COVID19) | Palpitations, shortness of breath, chest tightness, presyncope, which led to New onset atrial fibrillation with rapid ventricular response and required synchronized cardioversion and hospitalization. Discharged on anticoagulation and beta-blocker. | |
| COVID19 VACCINE (COVID19) | 12/23- began to experience intermittent right lower quadrant pain in the morning, fever of 100.4 F in the evening which subsided with ibuprofen. 12/24- no fever noted but intermittent right lower quadrant pain continued, seen at the Health Clinic, sent to Hospital ER for CT scan, diagnosed with appendicitis, appendectomy performed. | |
| COVID19 VACCINE (COVID19) | 8:27 Hives, itching all over chest and on tongue, tongue swelling, slight difficulty swallowing. 8:30 epi administered/ benedrly 8:32 pseudo seizures begin off and on till the 27th | |
| COVID19 VACCINE (COVID19) | 12/20/2020 12:00 PM DEVELOPED LAPID ATRIAL FIB. WENT TO ER WHEN IT PERSISTED, MEDICAL CENTER. ADMITTED TO INPATIENT; 12/21/2020 – ELECTRICAL CARDIOVERSION. MONITORED OVERNIGHT. 12/22/2020 – DISCHARGED *ON CHRONIC BLOOD THINNER | |
| COVID19 VACCINE (COVID19) | right after the vaccine she felt light headed felt better in observation after about 7 minutes employee c/o heart racing,Chest pressure, feeling light headed, throat scratchy and tight. allergy to MRI contrast dye only – Gadolinium. Has had lots of vaccines in the past without problems. Taken to ED via W/C was talking all the way not SOB admitted to ED. 12-28 States she was admitted to the hospital overnight for anaphalaxis on a second trip to ED. She will not be able to get her second dose of the vaccine. this should be entered into the VAERS reporting system. She is till using the benedryl. | |
| COVID19 VACCINE (COVID19) | 15 minute post vaccination observation patient denied any symptoms. Later in the day patient experienced significant nausea and vomiting followed by mild SOB and throat swelling. | |
| COVID19 VACCINE (COVID19) | Numbness in sole of feet. Unable to walk, develop high fever, resp failure resulting in intubation, acute kidney injury | |
| COVID19 VACCINE (COVID19) | Noted tongue starting to swell on 12/24 at 1030. Started on left side, then progressed to right side. No SOB, difficulty swallowing or breathing, but staff noted difficulty understanding her speech. Presented to ED at 1300. 50mg Benadryl given IV on 12/24 at 1328 and 125mg solumedrol given IV at 1327. Pt reported improvement in tongue swelling at 1630. | |
| COVID19 VACCINE (COVID19) | Pediatrician working in the hospital. Was exposed the an office contact wo had covid. Shoulder in soreness. At work on Wednesday. Felt lightheaded had to sit in chair. That’s all he reminders. He workup to a CODE team putting oxygen on him. He has a seizure. Took the COVID test has COVID. Admitted to hospital for 2 days. Likely a syncopal event. | |
| COVID19 VACCINE (COVID19) | 41-year-old male who presents to the ED today with a complaint of weakness in his bilateral arms and legs. He states he fell slightly weak yesterday but this morning when he woke up around 6 AM he was not able to get out of bed because he was so weak. He states he feels like he has no muscle strength in his arms and legs. He denies any fever. He denies any cough or shortness of breath. He denies any chest pain or abdominal pain. He denies any nausea, vomiting or diarrhea. He denies any numbness in his extremities. He denies any neck or back pain. He did receive the first Covid vaccination on December 17. | |
| COVID19 VACCINE (COVID19) | TTP; This is a spontaneous report from a non-contactable pharmacist. A 22-year-old male patient received the first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 mRNA VACCINE), intramuscular on 17Dec2020 as a single dose for COVID-19 immunization. The patient did not have any known relevant medical history. The patient had no allergies to medications, food or other products. Prior to the vaccination, the patient was not diagnosed with COVID-19. The patient’s concomitant medications were not reported. It was unknown if the patient received any other vaccines within four weeks prior to the vaccination. On 21Dec2020, the patient experienced thrombotic thrombocytopenic purpura (TTP); which was serious for hospitalization. The clinical course was as follows: The patient went to the emergency room/urgent care and was admitted in the early morning of 21Dec2020 due to TTP. Work-up was ongoing with no known results. On 21Dec2020, the patient also had a COVID-19 test which was negative. The patient was treated with unspecified corticosteroids and platelets. The clinical outcome of the TTP was unknown. The reporter assessed that it was unknown if the TTP was related to the vaccination. The lot number for the vaccine, BNT162B2, was not provided and will be requested during follow up.; Sender’s Comments: Current limited information does not allow a full medically meaningful assessment, especially lack of medical history, concomitant medications, concurrent illness and diagnostic workups such as coagulation test, Combs test, bacterial/virologic/immunological biomarkers to identify the etiology. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | Patient had mild hypotension, decreased oral intake, somnolence starting 3 days after vaccination and death 5 days after administration. He did have advanced dementia and was hospice eligible based on history of aspiration pneumonia. | |
| COVID19 VACCINE (COVID19) | “pancreatitis; acute lower abdominal pain; This is a spontaneous report from a contactable pharmacist. A 46-year-old non-pregnant female patient received the first dose of the bnt162b2 (PFIZER-BIONTECH COVID-19 MRNA VACCINE; Lot Number: EJ1685), intramuscularly on 18Dec2020 at 08:00 (as reported) at 46-years-old at a single dose for COVID-19 immunisation. The patient medical history was not reported. The patient had no known drug allergy (NKDA). Concomitant medications included acetaminophen (MANUFACTURER UNKNOWN), propranolol (MANUFACTURER UNKNOWN), sertraline hcl (MANUFACTURER UNKNOWN), sertraline hydrochloride (ZOLOFT); all taken for an unspecified indication from an unspecified date to an unspecified date (which were received within two weeks of vaccination). On 18Dec2020 at 17:00, the patient experienced pancreatitis and acute lower abdominal pain; which required hospitalization and were assessed as medically significant. The patient was hospitalized for pancreatitis and acute lower abdominal pain for 3 days on unspecified dates. The clinical course was reported as follows: The patient received the vaccine “” at some point in the AM on 18Dec2020 (as reported).”” That evening, the patient presented to the emergency department (ED) with acute lower abdominal pain. The patient was diagnosed with pancreatitis and was admitted overnight. It was unknown if the patient received any other vaccines within four weeks prior to the COVID vaccine. Prior to the vaccination, it was unknown if the patient was diagnosed with COVID-19. Since the vaccination, the patient had not been tested for COVID-19. Therapeutic measures were taken as a result of pancreatitis and acute lower abdominal pain. The clinical outcome of the events was recovering.; Sender’s Comments: The information currently provided is too limited to make a meaningful medical assessment. Other than a temporal association , there is no evidence or argument to suggest a causal relationship between BNT162B2 and the events pancreatitis and acute lower abdominal pain. The events are likely due to an underlying medical condition. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to RAs, Ethics Committees, and Investigators, as appropriate.” | |
| COVID19 VACCINE (COVID19) | Admitted to the hospital with hypertensive basal ganglia bleed, had a head bleed; This is a spontaneous report from a contactable consumer. A male patient of unspecified age received first dose of bnt162b2 (PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration in 2020 at single dose for COVID-19 immunization. Medical history included hypertension. The patient’s concomitant medications were not reported. After vaccination, the patient was admitted to the hospital with hypertensive basal ganglia bleed, had a head bleed in 2020. The outcome of event was unknown. Information on the lot/batch number has been requested. | |
| COVID19 VACCINE (COVID19) | resp distress; This is a spontaneous report from a non-contactable consumer (patient). An elderly male patient received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration at 16Dec2020 12:00 pm at single dose for covid-19 immunization. Vaccine location was right arm and it was the first dose. The patient medical history and concomitant medications were not reported. No known allergies. Patient was not diagnosed with COVID-19 prior to vaccination. The patient didn’t receive any other vaccines within 4 weeks prior to the COVID vaccine. The patient experienced respiratory distress on 19Dec2020, he was hospitalized for three days. Patient received treatment for the adverse event. Since the vaccination, the patient has been tested for COVID-19 with nasal swab on 19Dec2020, it was negative. The action taken in response to the events for BNT162B2 was not applicable. The outcome of events was unknown. The event was serious, the seriousness criteria was Caused/prolonged hospitalization. No follow-up attempts are possible; information about lot/batch number cannot be obtained. | |
| COVID19 VACCINE (COVID19) | Weakness and tingling down left arm; Weakness and tingling down left arm; Lightheaded; PVC’s every 3 beats; emotional too and just very tired; Can not read the vaccination card as she does not have her glasses; Palpitations; Fatigue; Slept a lot; Thready pulse and vertigo; Thready pulse and vertigo; Soreness in left arm at the injection site and down the left arm; Soreness in left arm at the injection site and down the left arm; This is a spontaneous report from a contactable nurse (patient). A 54-year-old female patient received first dose bnt162b2 (PFIZER-BIONTECH COVID-19 VACCINE, reason for no lot number of COVID Vaccine: Can not read the vaccination card as she does not have her glasses, Expiry Date unknown), via an unspecified route of administration in the left arm on 18Dec2020 at single dose for ‘Work with COVID patients’. Medical history included none. There were no concomitant medications. The patient experienced weakness and tingling down left arm (hospitalization) on 22Dec2020, lightheaded (hospitalization) on 22Dec2020, PVC’s every 3 beats (hospitalization) on 22Dec2020, soreness in left arm at the injection site and down the left arm on 18Dec2020, thready pulse and vertigo on 19Dec2020, fatigue on 20Dec2020, slept a lot on 19Dec2020, palpitations on 21Dec2020. Details as follows: Caller says she received the vaccine, she is a nurse. She got the vaccine on Friday, 18Dec2020. She had soreness in her arm and at the injection site on Friday but that was it. On Saturday (19Dec2020) she noticed a thready pulse, but went on with her day with only a little arm pain. Sunday (20Dec2020) she was fatigued and the thready pulse continued. She slept a lot on Saturday (19Dec2020) and Sunday (20Dec2020). Yesterday (21Dec2020) she felt a little better, but had palpitations here and there. This morning (22Dec2020) she went into work, was very lightheaded, had tingling down her left arm, and had palpitations. So she hooked herself up to a monitor. Her pulse ox was between 97-99%. Her heart rate would be in the 90s and then drop to 48, so she went down to the ED. She has had a CT, and she is throwing PVC’s every 3 beets. She has not been admitted as they are still waiting for results. She is still in the ED. They did a CT to see if there was a possible clot. On 18Dec2020 she received the vaccine around 2 PM. She had soreness at the injections site and down the left arm, which went away by Sunday (20Dec2020). She now (22Dec2020) has weakness and tingling down the left arm. It was never red or anything at the injection site. Saturday, 19Dec2020, she had thready pulse and Vertigo which lasted until Sunday 20Dec2020. She would be laying in bed and try to flip to the other side and having vertigo. When the fatigue started on Sunday (20Dec2020) she did not feel like herself. She was very emotional too and just very tired. Since she went to the ED she has had a CT scan, one with contrast and one without. She had a chest X-ray, and she is on a cardiac monitor. Results are pending. She has Trigeminy PVCs. She says she never goes to the hospital. But she is not admitted yet (pending clarification). Can not read the vaccination card as she does not have her glasses. Unable to read off the NDC, lot, and expiration date. History: Has been on the same vitamins for two years with nothing new. Blood pressure: Normal base line is 130s/80s maybe lower. Heart rate: Currently within her normal limits of 80s-90s. Depending on what happens, it was asked if she should get the second dose. The patient underwent other lab tests and procedures which included blood pressure measurement: 163/76 on 22Dec2020, chest x-ray: unknown result on 22Dec2020 (Result: Pending), computerised tomogram (CT scan): unknown result on 22Dec2020 (Result: Pending), heart rate: 80s-90s on 22Dec2020, Pulse oximetry: 97-99 % on 22Dec2020, cardiac monitor: results are pending on 22Dec2020. The outcome of events weakness and tingling down left arm, pvc’s every 3 beats, lightheaded, palpitations and fatigue was not recovered. The outcome of the event soreness in left arm at the injection site and down the left arm was recovered on 20Dec2020. The outcome of the events thready pulse and vertigo was recovered on 20Dec2020. The outcome of the event slept a lot was recovered on 20Dec2020. The outcome of other events was unknown. Information on the lot/Batch number has been requested.; Sender’s Comments: Based on available information, a possible contributory role of the subject vaccine cannot be excluded for the reported events due to temporal relationship. There is limited information provided in this report. Additional information is needed to better assess the case, including complete medical history, diagnostics, counteractive treatment measures and concomitant medications. This case will be reassessed once additional information is available. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | “low grade fever; Her blood pressure was high/ still really high/ blood pressure was up; headache; Fifteen to twenty minutes after she received the vaccine she became light headed and dizzy/ light headedness and dizziness; This is a spontaneous report from a contactable nurse (patient). A 36-year-old female patient received BNT162B2 (Lot#: EK5730) via an unspecified route of administration on 17Dec2020 afternoon at single dose in the left arm for COVID-19 immunization. Caller was unable to confirm the manufacturer of the vaccine that she received. It is not written on the card, and she didn’t see the vial. The patient medical history was not reported. Concomitant medications included oral contraception pill, but the name was unknown. Fifteen to twenty minutes after she received the vaccine on 17Dec2020 she became light headed and dizzy. She had to catch her breath. She couldn’t shake it off. The light headedness and dizziness lasted at that intensity for 10 minutes, but it never went away. They encouraged her to be admitted in the emergency room (ER). She would say that the seriousness of being light headed and dizzy was disabling. Caller didn’t remember the exact numbers for her blood pressure. It was 160’s over 105. Her heart rate was in the low 100’s, around 105. She stayed at the first monitoring station in the vaccine area for 2 hours. They were taking her blood pressure every five minutes. She was given diphenhydramine hydrochloride (BENADRYL) there and lots of water. After 3 hours and she was not improving they called a “”code medic”” that got the medical director and nursing supervisor to come. They encouraged her to go to the ER for continual monitoring. She stayed in the ER for4 hours and was given meds to help with the blood pressure. She was discharged from the ER home. She was nervous because of all this stemming from the vaccine. She had a low grade fever on 18Dec2020 (Friday) night. Caller stated her work had already reported her reaction. Occupational safety and the medical director are aware. Caller does not have reference number to provide. On 18Dec2020 (Friday) she was not overly concerned because it was the next day. Her blood pressure was high and her heart rate was in the 100’s. They monitored her for a couple of hours and she was given a diphenhydramine hydrochloride (BENADRYL). She went to the emergency room (ER) for a few more hours and received additional treatment. They sent her home to be monitored at home. She has been taking her blood pressure every day since and it had not come down. It was still really high. She called her primary care doctor. He was wanting her to start blood pressure for medication it. She was concerned about starting it with the assumption that it was related to the vaccine. She would like to know the right thing to do. It seems safe to take the medicine, but it was unknown that whether it was going to mask the blood pressure and something else be going on. On 18Dec2020 she still had a headache and didn’t feel well, but she thought she needed to give it some time. She had been anticipating not to feel well on 18Dec2020 (Friday). On 19Dec2020 she felt better considering she didn’t have a headache. On 19Dec2020 (Saturday) her blood pressure was 138/90 and she felt good. Then on 20Dec2020 she had the bad headache and her blood pressure was up. On 20Dec2020 (Sunday) she had a bad headache and her blood pressure was 156/100. She came to work today and her blood pressure had been high all day. She still had a headache and the light headedness continued. The outcome of the event low grade fever was unknown, of other remain events was not recovered.; Sender’s Comments: A causal association between BNT162B2 and the event dizziness cannot be excluded based on a compatible temporal relation. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to RAs, Ethics Committees, and Investigators, as appropriate.” | |
| COVID19 VACCINE (COVID19) | 10 minutes after the vaccination, she began clearing her throat, within 30 minutes began coughing, which led to chest tightness. Was evaluated in the ER and admitted for observation. Given: Prednisone 40mg po, Benadryl 25mg po Duoneb x 3 and Pepcid 20mg | |
| COVID19 VACCINE (COVID19) | Patient was found slumped over in wheelchair, drooling and unable to respond/follow simple instructions; sent to ED for evaluation. CT scans and MRI NEGATIVE for new/recent stroke; resident slurred speech likely due to hypertensive urgency | |
| COVID19 VACCINE (COVID19) | SNEEZING, STUFFY NOSE, LIP SWELLING, TINGLING MOUTH, THROAT CLOSING, UVULA SWOLLEN 1. Allergic reaction (Allergy, unspecified, initial encounter) . Pt. received Moderna COVID vaccine the morning of 12/22/20 and developed angioedema reaction the afternoon of 12/23 Vaccine is only new medication reported by pt. – denies any new products, foods, etc History of hives with spironolactone 2.5 months ago; taking HCTZ now – unknown if reaction is related to this med rather than vaccine? Due to severity of her reaction and uncertainty if all attributable to vaccine versus some other culprit I would recommend Epi pen at time of discharge Has received IM epinephrine, racemic epi neb, Solu-Medrol, Benadryl, Pepcid in the ED Continue with Benadryl 25mg IV q6h, Pepcid 20mg IV q12h, and Solu-Medrol 40mg IV q6h 2. Angioedema (Angioneurotic edema, initial encounter) . Arrived to the ED with R. side lip, tongue, and uvula and oral cavity edema Airway patent, room air O2 sats WNL – monitor throughout the night for any worsening of angioedema or airway involvement Treatment as above | |
| COVID19 VACCINE (COVID19) | Difficulty breathing 5 minutes after receiving first dose of Covid-19 vaccine by Pfizer/BioNTech, small erythematous spots to bilateral arms. | |
| COVID19 VACCINE (COVID19) | 33 y.o.ámaleáwith no significant past medical history except for obesity who has been working as a nurse in the emergency room department in our hospital and today he received COVID-19 vaccine and 30 minutes later patient started having increased saliva, cold hands and feet, left-sided pressure-like headache and some numbness in his legs at the same time he suddenly started talking only in first language and lost his ability to speak in second language. áHe understand second language but replying first language stating that he is talking in second language. áOn exam he was alert oriented confused by people not understanding his second language stating that his numbness and cold feeling in the hands and feet have improved. áInitially patient received 10 mg of Decadron for possible allergic reaction he had a head CT scan that was negative and his labs were remarkable only for hypokalemia. áPatient had no prior history of any neurological symptoms he was advised admission to the hospital for observation á á Patient symptoms resolved next day,he is alert oriented able to communicate in second language he had a head MRI and head neck MRA that came back negative and had an EEG that showed no seizure activities. Patient was seen in neurology consultation who felt that patient most likely had an episode of migraine headache. Patient is going to be discharged home and to have a follow-up with his primary care physician next week á | |
| COVID19 VACCINE (COVID19) | Patient reports taking Pfizer Covid vaccine and 2 hours after that she reports feeling not well. She recorded that one of the side effects was heart arrhythmias and hence she had her coworker checked her rhythm and she reported that her heart rate was in 180s and hence she was brought to the emergency department for further evaluation. She reports at the time she had palpitations and felt mild lightheadedness and dizziness. She was found to be in SVT with heart rate in the range of 1 80-220 and she received 1 dose of 6 mg Adenoscan after which she converted to normal sinus rhythm. At the time of my evaluation she is in normal sinus rhythm with heart rate in the range of 90-100. She denies any further palpitations. She reports she had chest tightness for the last 3 days which was assumed to be secondary to asthma and for which she was prescribed prednisone. Currently with the prednisone she does not feel any further chest tightness. She denies any chest pain shortness of breath, fever or chills. She reports remote history of arrhythmia following her foot surgery in the past however does not recall what arrhythmia she had at that time. | |
| COVID19 VACCINE (COVID19) | Patient presents to the emergency department 12/26 complaining of dry cough associated with fever and chills and headache associated with myalgia and diarrhea for 1 week duration. She had Covid vaccine 12/20 and the symptoms started the same night, she denied any sick contacts at home however she works at the Covid unit and reports constant exposure to sick Covid patients. | |
| COVID19 VACCINE (COVID19) | “Approximately 7 hours after receiving the vaccine patient who is a L&D Nurse return to work in her area. She describes that after finishing with a C-section she felt burning in both of her eyes (she thought this feels like an allergic reaction, but I am not sweating and haven’t rubbed my eyes). She went to the restroom to get a cloth to wash her eyes; afterwards she reports her vision went totally black in both eyes. She reports feeling frustrated that no one came to help and some panic in trying to figure out how to get out of the restroom. She did make it out of the bathroom. Her Staff reports she postured and turned arms inward, head going to one side and passed out. They also report ~ 10 minutes of incoherent conversation and stating “”I got the vaccine, maybe I was given the wrong thing and now I’m blind””. Upon waking, patient vision fully restored and patient does not remember incoherent conversation. Differential diagnosis- TIA vs. CVA > seizure disorder>>> complex migraine” | |
| COVID19 VACCINE (COVID19) | she is better but still not good; not to be able to breath; sore right arm; This is a spontaneous report from a contactable nurse (patient herself). A 62-year-old female patient received bnt162b2 (BNT162B2, lot EK5730), intramuscular on 18Dec2020 at single dose for immunisation. Medical history included asthma (hospitalized on Jan2020 and has not had any issues since that time, referring to her asthma) diabetes, high blood pressure, swelling, sciatica, blood cholesterol abnormal, rosacea, reflux, allergies, sinus congestion, shingles and post carpal tunnel surgery. Concomitant medications included lisinopril, hydrochlorothiazide, gabapentin, rosuvastatin, metformin, glipizide, doxycycline, sucralfate, cetirizine hydrochloride (ZYRTEC), pseudoephedrine, ascorbic acid, ergocalciferol, nicotinamide, retinol, riboflavin, thiamine hydrochloride (VITAMINS) and tramadol. The patient reported that she not to be able to breath (seriousness criteria-life threatening) on 22Dec2020. She woke up this morning and could not breathe and there was no reason for her to not be able to breath. She thought she may have had a reaction to the COVID vaccine. It was the only thing she could think of that might have caused her not to be able to breathe this morning. As treatment for not to be able to breath, she used Budesonide and Levosalbutamol in her nebulizer. She had sore right arm on 18Dec2020. She informed that she had done everything she can and she was better but still not good. She planned to take the second dose of the COVID Vaccine because she thought it was more important to be protected. She suspected that the vaccine was related to the events sore right arm and could not breathe. The outcome of the event not to be able to breath was recovering; for sore right arm was recovered on unknown date in Dec2020; for she is better but still not good was unknown.; Sender’s Comments: Severe allergic reaction including anaphylaxis is the known risk factor; a possible causal association between administration of BNT162B2 and the onset of not being able to breath cannot be excluded, considering the plausible temporal relationship and the known adverse event profile of the suspect product. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. |
| COVID19 VACCINE (COVID19) | positive COVID-19 test with symptoms; positive COVID-19 test with symptoms; Soreness at injection site; This is a spontaneous report from a contactable nurse (patient). A 40-year-old male patient received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration on 18Dec2020 17:30 in the left arm at single dose for COVID-19 immunization. There was no medical history or concomitant medications. The patient experienced soreness at injection site, cough, body aches on 19Dec2020; sore throat, voice changes from coughing on 20Dec2020; tested positive for covid on 21Dec2020; mild low congestion, loss of taste and smell on 22Dec2020. The nurse stated that he got the vaccine on Friday (18Dec2020). The next day (19Dec2020) he had common side effects: Soreness at the injection site and body aches, which were expected. He also had a cough on top of that, which progressed to the next day. His body aches and coughing were infrequent. The afternoon of Sunday (20Dec2020), he developed sore throat. Yesterday(21Dec2020), he said he could not work because he was still coughing and had a sore throat. His voice was also changing due to the coughing. He was getting better now. The doctor from Employee Heath said that the cough was concerning so he got a COVID swab test yesterday(21Dec2020), and today (22Dec2020) it came back positive. This morning (22Dec2020) he had loss of taste and smell. He no longer had sore throat or cough. He had the vaccine before the test. He wanted to know where they were at with information on this. Was this being monitored? How did this happen? Was it possible that the test was a false positive because he had the vaccine prior? He would like someone to give him an answer, if the test was a false positive due to the vaccine? His doctor could not tell if the test was legit a positive because of the vaccine. He was not able to work right now. He did not even know if the COVID was from the vaccine or not. Will he get compensation for this? Will his workplace cover his absences? In the case he went to the hospital, will this be considered a work related or vaccine related issue? The outcome of event soreness at injection site was recovered on 21Dec2020. The outcome of event tested positive for COVID was not recovered. The nurse considered the cough was disabling as this was not part of the symptoms to watch for after getting the vaccine. All of the symptoms currently besides the cough are not serious as of now, but it has put him out of work. The nurse considered all other events as non-serious except for cough (disabling). Information on the lot/batch number has been requested.; Sender’s Comments: Based on the information currently available, a lack of efficacy with BNT162B2 in this patient might not be completely excluded. | |
|---|---|---|
| COVID19 VACCINE (COVID19) | anaphylaxis; This is a spontaneous report from a contactable physician reporting on behalf of patient. A patient of unspecified age and gender received single dose of BNT162B2 (batch/lot number and exp date not reported), via an unspecified route of administration on an unspecified date for immunisation. The patient’s medical history and concomitant medications were not reported. On an unspecified date, the patient experienced anaphylaxis with a very protracted course requiring an epi dose for 4.5 days and was still in the ICU (date/s unspecified) following administration of the COVID vaccine. The physician would like to use a drop of leftover vaccine from one of the vials to do a future skin test after the patient is stable. They were unsure if they needed permission as this was standard practice in allergy to test afterwards but wanted to check in with the company. The outcome of event was unknown. Information about batch/lot number has been requested.; Sender’s Comments: A possible causal association between administration of BNT162B2 and the onset of anaphylaxis cannot be excluded, considering the plausible temporal relationship and the known adverse event profile of the suspect product. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | I received my vaccine on 12/22 around noon. I woke up at 4am this morning 12/25 with tingling in my lips and tongue, in addition to the left side of my face being swollen. Not entirely sure what these side effects are from exactly but thought it best to report my experiences I was hospitalized on 12/25 to 12/26 because my reaction did get worse. I was experiencing numbness on the left side of my body and swelling, numbness, and redness on the left side of my face as well. The hospital didn?t really give any explanations but I?m only now have mild swelling and numbness on the left side of my face. Everything else has resolved at this point in time. | |
| COVID19 VACCINE (COVID19) | Resident found unresponsive in her room. Note from earlier: Resident appears to be weak today. Resident ate a few bites of dinner before refusing the tray. Writer encouraged fluids. Vitals 123/72 80HR BS 166. Will log for Doctor and continue to monitor. Was sent out 911. | |
| COVID19 VACCINE (COVID19) | I received the vaccine shot on 12/23/20 at 3 pm. By 12/24/20, I had pain in my left arm that radiated to my neck and back on the left side. Over the past 4/5 days, I continue to have pain in my left arm, back and neck | |
| COVID19 VACCINE (COVID19) | Adverse reaction post Covid vaccine. Waited for 20 min post vaccine. Experienced S/S Heart palpitations, shortness of breath, tingling in extremities, diaphoretic after leaving clinic observation. Drove back to hospital, escorted by pre surgical testing hospital staff and taken by wheelchair to ED. | |
| COVID19 VACCINE (COVID19) | Was given the vaccine and about 5 minutes later started having swelling and my eyes and face. It was watched for a few minutes and was assessed by EMS and taken to the emergency department. I was given epinephrine, Benadryl, Solu-Medrol, Pepcid, IV fluids, DuoNebs and observed overnight. I was given multiple rounds of Benadryl, steroids, Pepcid, DuoNebs | |
| COVID19 VACCINE (COVID19) | Subject received vaccination Wednesday Dec 16th in the afternoon. He became symptomatic (shortness of breath, low grade fever) the next day. Went to the Emergency room on Saturday Dec. 26th, 2020 due to shortness of breath, had an 02 Sat of 60%, and was hospitalized in the ICU at another hospital (due to bed unavailability). | |
| COVID19 VACCINE (COVID19) | Received vaccine at 1:30 pm yesterday, noted onset of symptoms at 8:45 pm. Numbness and tingling to mouth and bilateral upper and lower extremities, mild vision change, feeling of some swelling to bilateral eyelids. Also swelling to lips. She also did take zinc gluconate 50 mg last night and this morning. Has never taken zinc 50 mg, but has taken zinc as component of multivitamin/pre-natal vitamins. Patient was prescribed Pepcid 20 mg BID, Medrol 4 mg dose pack 21 pill taper until complete. Also given Benadryl 25 mg – 50 mg every 4 – 6 hours for allergy symptoms. And provided with an Epi-Pen for home. | |
| COVID19 VACCINE (COVID19) | Rushed to ER. Has now been tubed and put into the ICU and has had full-cardiac arrest less than 24 hours after receiving the vaccine. | |
| COVID19 VACCINE (COVID19) | Hospitalized 12/29, has now been tubed and put into the ICU | |
| COVID19 VACCINE (COVID19) | Patient observed for 15 minutes in the clinic after vaccine with no issues. Patient is a NP and left clinic. 10 min after leaving, was in physician lounge and had tachycardia, dizziness, flushing. Hospitalist in lounge recorded pulse as 180 normal rhythm. Patient taken to ED. In SVT – heart rate eventually reduced. Patient released on cardiac monitor to home. Later that night, patient had increased heart rate again – 160’s – while in bed. Admitted to hospital. | |
| COVID19 VACCINE (COVID19) | Chest pain, short of breath. Morphine, sublingual Nitro, IV Nitro drip & Heparin drip | |
| COVID19 VACCINE (COVID19) | Hospitalization 12/26 for Covid PNA | |
| COVID19 VACCINE (COVID19) | Client was vaccinated approx 10:00am at the Health Dept. Client left after 15min without incident. Client went to report to work and began feeling nauseated, dizzy, and feeling as if was going to faint. Client returned to Health Department approx 12:30. Client was diaphoretic, HR was 140bpm, and BP was 178/120. Client was given 50mg/20mL of diphenhydramine PO and EMS was called and client taken to local ER. Client remained A+Ox3 at time of EMS transport. Spoke with client evening of 12/28/2020. He reports he was transferred to another facility and was told was in a-fib. He reports that he is feeling much better but heart still fluttering. Spoke with client afternoon of 12/29/2020. He reports that he is being discharged in a few hours and is being sent home on metoprolol and a baby ASA. He reports they had been working on getting his BP down. He reports that he is feeling better and is going to be scheduled for a heart cath outpatient. He reports that he was initially in a-fib, then a-flutter, and then arrhythmia. He reports that he has been seen by Dr. a Cardiologist, while hospitalized. | |
| COVID19 VACCINE (COVID19) | Monitored x 15 min per guidelines. Began to experience SOB and throat swelling, after which pt presented to the ED for tx, dx acute hypertensive urgency with severe hypertension. | |
| COVID19 VACCINE (COVID19) | Itching, cough. Given benadryl 50mg and epinephrine 0.3 in vaccine clinic, and taken to ED for further tx. | |
| COVID19 VACCINE (COVID19) | With five minutes of vaccine Increased heart rate shot up to 198 bpm. Regular resting heart rate for me is in the 50s. Left pupil dilated completely, right people constricted to pin size. Extremities went numb and ice cold. Went to emergency room Per request of EMTs at vaccination center. Was given high-dose Benadryl and Saline IV. Heart rate return to normal and all other symptoms disappeared after treatment. | |
| COVID19 VACCINE (COVID19) | Difficulty catching my breath, stiff joints in legs | |
| COVID19 VACCINE (COVID19) | “Patient was monitored for >15 minutes after vaccination. Patient told a nurse that her knees felt weak. Patient then fainted and was laying on the floor when i arrived. Patient reported she felt like she was “”floating”” and she did not want to “”fall””. She was also nausea and wanted to vomit and did not end up vomiting anything up. Patient fainted several more times. Her BP was around 143/80 and unsure about the pulse. Patient then become unresponsive for 20-30 seconds.” | |
| COVID19 VACCINE (COVID19) | Fever, coughing, tachypnea, tachycardia | |
| COVID19 VACCINE (COVID19) | Received vaccine at 13 15. At 1351 c/o mild chest pain. 1353 c/o throat fullness and anxiety; 1354 bp 148/81 HR 99 O2 sat 100% c/o difficulty swallowing, SOB and itching. Rapid response called. Benadryl 50 mg IVP given at 1355. 1356 Solumedrol 125mg given IV; 1357 Epinephrine given; 1358 Pepcid 20 mg given. 1400 Normal saline 250 mg IV; 1415 transported by ambulance to local ER. In waiting area in local ER no attention. Came back to Hospital and admitted to observation | |
| COVID19 VACCINE (COVID19) | 10 MINUTES FOLLOWING VACCINE – SOB, COUGH, TIGHTNESS IN CHEST, THRAOT SWELLING, DIFFICULTY SWALLOWING, LIGHT HEADEDNESS, AND ELEVATED HEART RATE. ORAL AND IM BENADRYL ADMINISTERED, 2 DOSE OF EPINEPHRINE, 2 NEB TREATMENTS, O2 PLACED. 911 CALLED AND TRANSPORTED TO EMERGENCY FOR FURTHER TREATMENT AND MONITORING. AT HOSPITAL IV STEROID ADMINISTERED. SYMPTOMS SUBSIDED WITH SECOND DOSE OF EPINEPHRINE, HOWEVER RETURNED 3 HOURS LATER AND ANOTHER DOSE OF BENADRYL ADMINISTERED. ELEVATED HEART RATE CONTINUED AND IV FLUIDS ADMINISTERED TO ATTEMPT IN BRINGING DOWN HEART RATE. IV FLUIDS WERE NOT EFFECTIVE. HEART RATE (118-120) REMAINED ELEVATED INTO THE OVERNIGHT HOURS AND SUBSIDED AROUND 1:30A ON 12/29/2020. CONTINUED HEADACHE, NAUSEA ONSET, FATIGUE, DIFFICULTY SWALLOWING AND COUGH ON 12/29/2020. | |
| COVID19 VACCINE (COVID19) | The morning after getting vaccine, patient had high BP 213/159, 92% on room air, shaking, chills, LCTAB with diminishment at bases, emesis. Admitted to hospital for pneumonia. | |
| COVID19 VACCINE (COVID19) | Vaccine administered with no immediate adverse reaction at 11:29am. Vaccine screening questions were completed and resident was not feeling sick and temperature was 98F. At approximately 1:30pm the resident passed away. | |
| COVID19 VACCINE (COVID19) | headache, nausea, light headed, swelling of the hands Patient was given 1000mg of acetaminophen and 25mg diphenhydramine. Patient was monitored for 60 minutes. | |
| COVID19 VACCINE (COVID19) | Pt. developed tachycardia, hypertension and felt weak with decreased verbal responsiveness, alert but lethargic. She complained of dry throat, took a sip of water then began persistent coughing and wretching also C/O itching of her throat. She denied difficulty breathing, there were no cutaneous signs of edema, tongue enlargement, etc. | |
| COVID19 VACCINE (COVID19) | Pt. began to feel weak with palpitations about 8-10 minutes after vaccination, her pulse was extremely fast, she then began to complain of lower mid-esophageal burning | |
| COVID19 VACCINE (COVID19) | Pt developed anaphylaxis, was given IM Benadryl, and was sent to the ED. Pt spent 1 night in the hospital, went home, and has come back and is in the ICU. Pt had hives, itching, chest tightness, swollen lips. | |
| COVID19 VACCINE (COVID19) | Shortness of breath Hives, Fever, chills joint pains. I was Tx in the ER with Pepcid IV Solumedrol IV, Benadryl IV ,Tylenol and IV fluids. I was discharged on prednisone , Benadryl and Tylenol. | |
| COVID19 VACCINE (COVID19) | Approximately 15 minutes after IM injection, patient developed chest tightness. Patient was taken to the Emergency Department for treatment. Was given sublingual nitroglycerin and subsequently admitted to the hospital for observation. Patient discharged home the following morning in good condition. | |
| COVID19 VACCINE (COVID19) | My grandmother died a few hours after receiving the moderna covid vaccine booster 1. While I don?t expect that the events are related, the treating hospital did not acknowledge this and I wanted to be sure a report was made. | |
| COVID19 VACCINE (COVID19) | About 3 am after the injection I woke up with severe chest pains and headache and went to the ER. I was admitted for 2 days and was released with a prescription of Isosorb Monoer, Metroprol, aspirin which is used as a blood thinner. The diagnosis and prognosis was severe migranes and cardioartery disease. | |
| COVID19 VACCINE (COVID19) | Left side of my face went numb. 5 minutes later tongue was dry and tingly. Went back to place of work and went to monitoring room. Went to ER to be checked out. Got better over that day. I was admitted the same day and discharged the next day. | |
| COVID19 VACCINE (COVID19) | anxiety, tachycardia, flushing, diaphoresis, HTN, SOB | |
| COVID19 VACCINE (COVID19) | Patient administered Pfizer-BioNtech vaccine, dose #1 in series at 810AM, without notable concerns for 10 minutes. At 10 minutes post vaccination, patient developed itching and some blotching, throat becoming scratchy. No known allergies to components listed in vaccine, though does have a listed allergy to contrast dye, does not carry an epi-pen. Patient was walked to urgent/emergency care in the clinic, where she was seen immediately. Patient was given diphenhydramine 25 mg IV, pantoprazole 40 mg IV, with mild uticaria continuing. Patient notes she feels her ‘back is on fire’. Patient was then given methylprednisolone 125 mg IV. At this time, no SOB is noted, uticaria is still present. 0930 patient reports swelling in throat and respiratory difficulties at this time with visible edema in the neck. 0.3 mg Epinephrine given at 0935, epinephrine drip and racemic epinephrine neb given. 1006 Patient on epinephrine drip 2.5 mics/hour. Patient transferred to Medical Center ICU, where she remains at the time of this report. | |
| COVID19 VACCINE (COVID19) | the patient died; This is a spontaneous report from a contactable consumer through a Pfizer employee. A 98-99 years old patient of an unspecified gender received bnt162b2 (COMIRNATY), via an unspecified route of administration possibly on 27Dec2020 at single dose for covid-19 immunization. The patient’s medical history and concomitant medications were not reported. The patient died on 29Dec2020. Event details: The Pfizer employee was informed, by a member of the Covid vaccine team at the ministry of health, that an elderly person 98-99 years old, who used to stay in an elderly home, who also had other serious diseases and received the vaccine possibly on 27Dec2020, had died this morning (29Dec2020). As it was mentioned to the Pfizer employee, they were ‘sure’ that the cause of death did not related to the vaccine. It was not reported if an autopsy was performed. Information on the lot/batch number has been requested.; Reported Cause(s) of Death: unknown cause of death | |
| COVID19 VACCINE (COVID19) | 10 minutes after receiving vaccination, a significant increase in HR was noted, along with a tingling sensation through out body. Also, scratchy throat was noted. Alert by patient made to staff at vaccination site. Sweating noted and shortness of breath at that time. Epi pen given via L thigh IM. PIV started and benadryl and solumedrol given. Relief of symptoms noted very shortly after Epi administration. Taken to ER for 4 hour observation. Sent home after 4 hours and given prednisone to be taken at home, 50mg daily for 4 days. No further adverse symptoms noted. | |
| COVID19 VACCINE (COVID19) | decreased range of motion in vaccinated arm: unable to raise left arm above shoulder x 72 hours now due to pain. no associated numbness or swelling. I am a surgeon and this impacts my work and driving. I would not have been able to operate during these last 3 days and while the pain is better 72hrs later, my arm is still out of commission. I think we need to inform healthcare providers who perform procedures that they may want to schedule the vaccine when no planned procedures for at least 72 hrs. Due to this issue I will be unable to proceed with the second dose, unless I can take it a week later than the scheduled January 24, 2021. It is taking too long to regain full function of the arm but i expect it will be back to normal as it is better today 72 hrs later. | |
| COVID19 VACCINE (COVID19) | Facial numbness radiating down left side of neck to left arm, elbow and chest. Went to ED, admitted for observation overnight. | |
| COVID19 VACCINE (COVID19) | Patient presented to the ER on 12/26/2020 with complaints of fever, chills and SOB for about 3 days. Stated it was a gradual onset and has been intermittent. Also reported some nausea and chronic loose stools, mild loss of smell and sinus drainage, non productive cough. Discharged to observation and then home on 12/27/2020. Patient readmitted to hospital on 12/30/2020. | |
| COVID19 VACCINE (COVID19) | Within 3 minutes of vaccination patient became fully flushed head and neck, with rapid heart rate (112), and feeling like her airways were tightening.. Nurse immediately called for response, administered Epipen, when response arrived applied oxygen and transported to ED. Solumedrol 125 mg, Bendadryl 25 mg, and Famotidine 20 mg, she responded well and was released home with Rx Prednisone 40 mg x 3 days. Only residual effect was a dry/sore throat. | |
| COVID19 VACCINE (COVID19) | 12/30 9:30 am developed angioedema. Swelling of face, lips, tight throat. Also had bright red rash over body trunk and arms. Both palms were red, hot and painful. | |
| COVID19 VACCINE (COVID19) | Weakness, fatigue, decreased appetite, upper extremity shaking, sternal red blotchy rash, decreased mental status, non-verbal, decreased level of conscious, mottling, left side facial droop, hypertensive, fever, unable to follow commands | |
| COVID19 VACCINE (COVID19) | headache 15 minutes after receiving vaccine, 3 to 4 hours later broke out in rash on upper extremities, ABD pain, itchy, and arm swelling, throat hurts. SOB | |
| COVID19 VACCINE (COVID19) | Left sided weakness, fatigue for 3 days post immunization. Patient was seen by health care provider on 12/30/2020. Provider transferred patient to Hospital ER for further evaluation. | |
| COVID19 VACCINE (COVID19) | At about 6am after receiving the shot I felt very tender at the injection site almost as if it was bruised. Then over the course of the day I started to feel very itchy all over my body but mainly on my right side. 10pm I got up for work, and could barely move, I’m feeling intense back pain on my lower back on the right side. I can walk but its all very limited motion, I can really only manage by putting all my weight on my left side. | |
| COVID19 VACCINE (COVID19) | Patient presents with ? Altered Mental Status ? Headache á á HPI Patient presents to ER by EMS ambulance after family called 911 as patient was incomprehensible with slurred speech and moaning on the phone this evening. On arrival of EMS patient was asleep in bed and reportedly unresponsive other that to localize to pain. EMS transferred patient to ER. On arrival to ER patient had GCS 7. Reportedly patient is locum nurse who works in a Nursing Home and patient reportedly received COVID vaccination 2 days ago and that night reportedly began complaining to family on the phone of headache, nasal congestion, sore throat, cough, fever, chills, nausea, emesis, myalgias, and lethargy. Per the medical record patient has history of seizures, migraines, and sciatica. No other information is known on patient arrival to ER. 1. Peripheral IV right dorsal hand placed by EMS in route to ER. 2. On arrival to ER GCS 7 (E1M5V1) and roving eye movements with episodic lateral conjugate and at times disconjugate gaze concerning for seizure activity. Arms and legs with moderately increased tone but no clonic movements and patient able to localize bilaterally. 3. Ativan 1 mg IVP for seizure, then further 2 mg IVP for persistent seizure. 4. Fosphenytoin 1,000 mg IVPB in ER for loading dose of antiseizure medication. 5. Patient had significant improvement following completion of Ativan 3 mg IVP and GCS improved to 14 (E3M6V5) from GCS of 7 (E1M5V1). 6. Patient able to communicate after improvement as above and reports she has had headache or migraine for past several days as well as dysuria with bilateral CVA pain and has significant pain on percussion of bilateral CVA and moderate pain on palpation of bilateral flanks. No nuchal rigidity or pain with ROM of neck. Additionally, she complains of severe headache and diffuse pain of back and abdomen/pelvis. She reports a history of seizures in the past and reports she had one last month and was treated at a hospital in her home state. She denies antiseizure medications. Additionally, patient reports nonproductive cough, sore throat, nasal congestion, fever, chills, myalgias, lethargy, nausea, and episodic emesis over the past 2 days. She has anterograde amnesia following seizure and does not recall events. 7. Normal saline 1,000 mL IV bolus, then 100 mL/hour in ER. 8. CT of head with and without contrast performed and negative for intracranial hemorrhage, lesions, stroke, or other acute pathology. 9. CT of chest/abdomen/pelvis with IV contrast shows no acute pathology or notable abnormalities. 10. Lactic acid drawn and normal. 11. UA and urine microscopy collected by straight catheterization and culture collected and pending. 12. Blood cultures x 2 collected and pending. 12. Rocephin 2 mg IVPB in ER after blood cultures collected. 13. Vancomycin 20 mg/kg (1,500 mg) IVPB following Rocephin. 14. Dexamethasone 10 mg IVP in ER. 15. Duoneb nebulizer in ER. 16. Called to discuss with patient’s daughter and the family’s preferred contact who is 23 years old. She reports patient had COVID vaccination 2 days ago and beginning that night patient has complained of headache, fever, chills, nonproductive cough, sore throat, nasal congestion, myalgias, and lethargy. Additionally, she reports patient has history of seizures on at least 1 occasion in the past a few months ago but is not on antiseizure medications. She reports patient had MRI and MRA of her brain at that time and reportedly and intracranial aneurysm was identified at that time. 17. Called to request transfer to another facility and spoke with hospitalist who states there is no neurologist there and suggests transfer to larger tertiary care facility with neurology. 18. Called to request transfer and accepted by ER provider. 19. Transfer by ALS ground ambulance with telemetry, pulse oximetry, O2 to keep > 02%, vitals every 30 minutes, normal saline at 100 mL/hour, Rocephin 2 grams IVPB, vancomycin 20 mg/kg (1,500 mg) IVPB in ER. á DISPOSITION Patient Stabilized and Transferred Data Unavailable Wed Dec 30, 2020 2:12 AM CST | |
| COVID19 VACCINE (COVID19) | Anaphalaxis reaction, stridor an unable to breathe. Happened in 30 seconds | |
| COVID19 VACCINE (COVID19) | Spouse awoke 12/20 and found spouse dead. Client was not transferred to hospital. | |
| COVID19 VACCINE (COVID19) | Resident in our long term care facility who received first dose of Moderna COVID-19 Vaccine on 12/22/2020, only documented side effect was mild fatigue after receiving. She passed away on 12/27/2020 of natural causes per report. Has previously been in & out of hospice care, resided in nursing home for 9+ years, elderly with dementia. Due to proximity of vaccination we felt we should report the death, even though it is not believed to be related. | |
| COVID19 VACCINE (COVID19) | Within 24 hours of receiving the vaccine, fever and respiratory distress, and anxiety developed requiring oxygen, morphine and ativan. My Mom passed away on the evening of 12/26/2020. | |
| COVID19 VACCINE (COVID19) | Near syncopal episode approximately 2.5 hours after vaccination. Sudden onset of dizziness, nausea, and diaphoresis. Was admitted to ED and observed overnight. Full cardiac work up was done and shown to be within normal limits. I have no pre-existing conditions and considered to be a healthy adult. | |
| COVID19 VACCINE (COVID19) | On Dec. 20, 2020 around 11:30 PM, 2 days after patient received her COVID-19 vaccination, she was found on the bathroom floor , obtunded, very pale, diaphoretic, nauseous, and complaining of severe chest pain. Paramedics was called and patient was transported to the nearest emergency room. According to paramedics, on the way to the ER while patient was in the ambulance,she was noted with a sudden drop in heart rate about 19 beats/minute and have to be given Atropine IV Push, oxygen and was connected to transcutaneous pacing which improves her heart rate. In the ER patient continued to have chest pain and she was given Morphine, Oxygen, Nitroglycerine and Aspirin. IM had an EKG which showed Sinus Bradycardia with a Right Bundle Branch Block. She had serial ekgs, a chest x-ray, laboratory testing which included Troponin. Her first Troponin level came back elevated prompting her hospital admission to Telemetry. Her next 2 Troponin level improved and return to normal range and her chest pain has resolved.. She underwent a Stress Test which came back negative. Patient was admitted for a total of 20 hours in the Telemetry unit with Cardiology consultation before being discharged home last . She was re-evaluated by the cardiologist yesterday which diagnosed her a chest pain of unknown origin. | |
| COVID19 VACCINE (COVID19) | RESIDENT CODED AND EXPIRED | |
| COVID19 VACCINE (COVID19) | Rash, Itching and swelling of left arm. Progressed to tachycardia in the 150’s, hypertension 200/114. Tingling of lips, dizziness | |
| COVID19 VACCINE (COVID19) | Went to Emergency room on 12/28/20 because he was short of breath. Tested positive for COIVD_19 and was admitted with hypoxia. | |
| COVID19 VACCINE (COVID19) | Injection given on 12/28/20 – no adverse events and no issues yesterday; Death today, 12/30/20, approx.. 2am today (unknown if related – Administrator marked as natural causes) | |
| COVID19 VACCINE (COVID19) | Death by massive heart attack. Pfizer-BioNTech COVID-19 Vaccine EUA | |
| COVID19 VACCINE (COVID19) | pt passed away with an hour to hour and 1/2 of receiving vaccine. per nursing home staff they did not expect pt to make it many more days. pt was unresponsive in room when shot was given. per nursing home staff pt was 14 + days post covid | |
| COVID19 VACCINE (COVID19) | pt was a nursing home pt. pt received first dose of covid vaccine. pt was monitored for 15 minutes after getting shot. staff reported that pt was 15 days post covid. Pt passed away with in 90 minutes of getting vaccine | |
| COVID19 VACCINE (COVID19) | The patient had COVID19 infection diagnosed 12/14/2020, and he stated 5 to 10 days after this, he developed shortness of breath. Had vaccine on 12/24/2020. Hypoxic and short of breath with COVID19 pneumonia on 12/29/2020. I do not know if this is an adverse effect or temporally related or if the vaccine activated prior infection. | |
| COVID19 VACCINE (COVID19) | “my left arm and breast were tender with some mild body aches and low grade headache/Arm soreness; my left arm and breast were tender with some mild body aches and low grade headache/ left breast was increasingly painful with swollen palpable lymph nodes; my left arm and breast were tender with some mild body aches and low grade headache/ left breast was increasingly painful with swollen palpable lymph nodes; my left arm and breast were tender with some mild body aches and low grade headache/ left breast was increasingly painful with swollen palpable lymph nodes; my left arm and breast were tender with some mild body aches and low grade headache; body soreness and stiffness; This is a spontaneous report from a contactable healthcare professional. A 34-year-old female patient received first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE, lot number: EH9899), via an unspecified route of administration on 18Dec2020 at single dose for COVID-19 immunisation. The patient medical history and concomitant medications were not reported. The patient stated, “”the day after the injection, my left arm and breast were tender with some mild body aches and low grade headache. Sunday Arm soreness went away but left breast was increasingly painful with swollen palpable lymph nodes, with body soreness and stiffness. Monday left breast is still remarkably tender with palpable lymph nodes”” on 19Dec2020 (reported as Seriousness criteria-Caused/prolonged hospitalization: Yes, on an unspecified date in Dec2020). The outcome of the event was not recovered.; Sender’s Comments: The reported information is limited. Based on the close temporal relationship and the description of the events, there is a reasonable possibility that the events are related to BNT162 vaccine. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to regulatory authorities, Ethics Committees, and Investigators, as appropriate.” | |
| COVID19 VACCINE (COVID19) | Itchy throat, red eyes after 30 minutes. EMS on site gave IV Benadryl, epi pen shot and took to ER for monitoring. Vitals were good so he was discharged. | |
| COVID19 VACCINE (COVID19) | 10 minutes after receiving vaccine, patient reported numbness across upper lip which progressed to her tongue. Felt tingling and dryness of tongue and swelling. No difficulty breathing or swallowing, no chest pain, no wheezing, no rash, no itching. Taken to ED and given methylprednisolone 125mg IV, diphenhydramine 50mg IV, famotidine 20mg PO. Patient improved and monitored x 4 hours with resolution of symptoms. Prescribed prednisone 50mg po x 4 days. | |
| COVID19 VACCINE (COVID19) | erythema, tachycardia, tachypnea, headache, uncontrolled dystonic shaking | |
| COVID19 VACCINE (COVID19) | Developed sudden onset of shaking chills and fevers as high as 103.0. She has developed a small circular 5 x 5 area of erythema and firmness at the injection site of her left upper arm. | |
| COVID19 VACCINE (COVID19) | pt received vaccine at covid clinic on 12/30 at approximately 3:30, pt vomited 4 minutes after receiving shot–dark brown vomit, staff reported pt had vomited night before. Per staff report pt became short of breath between 6 and 7 pm that night. Pt had DNR on file. pt passed away at approximately 10pm. Staff reported pt was 14 + days post covid | |
| COVID19 VACCINE (COVID19) | Received my vaccine on December 22nd, 2020 at around 830 Am. That afternoon, about 3 PM, I started to have a reaction. It started with tingling/numbness in my right hand which progressed up my arm into my elbow. About 10 minutes later, it then progressed into my right foot, and my left foot. About 10 minutes after that, I started to get flushed and a neck rash (diagnosed from Dr.). I took Benadryl and Ibuprofen 800mg PO every 6 hours for the next 24 hours. The numbness in my right foot and left foot along with the flushness went away a couple hours later. Although, the numbness in my right hand never went away. It came and went for the next 4 days until December 27th and 28th when it started getting worse. On December 28th evening, it got so bad that I was debating going to the emergency room around 1 am. The numbness and tingling was in my right hand and started shooting up my arm. The nerve pain around my wrist was unbearable. I finally fell asleep and the next morning, it was not nearly as bad, but was still there. The numbness and tingling moved from my right hand mainly to right hand, right foot, right leg, left foot and left hand today (12-30-2020). | |
| COVID19 VACCINE (COVID19) | angiodema. hospitalized overnight. During the hospitalization pt was started on an epi gtt and given MTP 125 mg. He had subsequent hyperglycemia, and increased his rate on his insulin pump to 2 U/hr (from 0.83 U/hr). Pt then decreased his rate to 1.4 U/hr while on the epi gtt, and then to 1.1 U/hr when off of the epi gtt. He also strengthened his carb ratio from 1:12 to 1:10 last night.Pt reports he had postprandial hyperglycemia overnight after his meal, but then BG corrected overnight. Pt reports fasting this morning is in the 110s. This morning’s breakfast on 1:10 CR with well controlled BG. | |
| COVID19 VACCINE (COVID19) | Resident received vaccine per pharmacy at the facility at 5 pm. Approximately 6:45 resident found unresponsive and EMS contacted. Upon EMS arrival at facility, resident went into cardiac arrest, code initiated by EMS and transported to hospital. Resident expired at hospital at approximately 8 pm | |
| COVID19 VACCINE (COVID19) | Due Date unknown/6-7 wks pregnant -nausea -Body felt heavy -headache -in & out consciousness -Fatigue, severe -Heart Racing | |
| COVID19 VACCINE (COVID19) | Patient had an anaphylactic reaction to the vaccine the day after it was given and went to the nearest ER. | |
| COVID19 VACCINE (COVID19) | Patient stated he stopped his blood pressure medications 3 days prior to vaccination due to a previous reaction to losartan, a medication he was no longer taking. Patient took aspirin and a MVI on day of vaccination and drank lemon water. Patient developed tingling sensation in his mouth after eating dinner around 18:00. Patient stated he ate tacos with apple cider and noticed tingling after dinner. Patient stated he took two benadryl with no relief. His tongue continued to swell and he took two additional benadryl at 22:00. Once he developed difficulty swallowing he went to the emergency department. Patient presented to the ED with tongue swelling and difficulty swallowing. At 23:57 he was adminsitered 0.3mg of epinephrine IM, diphenhydramine 25mg IV, famotidine 40mg IV, dexamethasone 10mg IV at 0114, methylprednisolone 60mg q6hrs started at 0417, diphenhydramine 25mg q6hrs IV started at 0416, albuterol 2.5mg via neb q6hrs started at 0710 | |
| COVID19 VACCINE (COVID19) | Patient died within 12 hours of receiving the vaccine. | |
| COVID19 VACCINE (COVID19) | Resident received vaccine in am and expired that afternoon. | |
| COVID19 VACCINE (COVID19) | Started feeling a reaction immediately after the vaccine, felt blurred vision, dizziness, racing heartbeat, chest rash and face, itching all over, difficulty swallowing, tongue tingling and wheezing. Sent to ED. EPI and Benadryl. 1800 Went to see her in the ED, room 33. She has red rash to neck, shaky hands itching to neck and chest. ED Dr to discharge, she stated husband to pick her up and she will follow up with OH tomorrow. ——————————————————————————————————————–RN ED gave her Epinephrine 0.3 mg, Methylprednisolone 125mg, Diphenhydramine HCL 50 mg, Zofran 4mg, Lorazepam 1 mg, Hydroxyzine HCL 50 mg Sumatriptan 6mg , Discharge from ED at 1902 —————————————————————————————————————————– RN 12/29/2020 1715 called to check on patient. left voicemail for her to call OH. ???????..? 12/29/2020 1838 left voicemail for patient to call OH. ??????????????????????. 12/30/20 2030 spoke with her. Tuesday 12/29 3pm-4pm dizziness, confusion, sob. Wheezing. Ambulance called. Hospital admitted. Intubated for less than 24 hours. Breathing treatments, epi drip. Now just on steroids and walking around and feeling better. Still admitted at hospital. Hoping discharged tomorrow. ————————————————————————–RN | |
| COVID19 VACCINE (COVID19) | Patient received dose 1 of COVID vaccine 12/24. He developed symptoms consistent with COVID infection on 12/25. He was seen in the emergency room at Hospital on 12/27,was diagnosed as COVID positive, and was discharged to home. He returned to the emergency room on 12/29 and was admitted to the hospital for treatment related to COVID infection. He is currently admitted to Hospital. | |
| COVID19 VACCINE (COVID19) | 10 minutes after vaccine numbness and tingling in left foot, hand and left side of face. Spacing out feeling. Feel like i’m going to pass out. | |
| COVID19 VACCINE (COVID19) | EDD – 4/1/2021 – Contractions at 26 w 3 days sent to L&D to be monitored on 12/28/20. Covid-19 Sars Vaccine given 1st dose 12/27/20. Patient was diagnosed with Fetal Hydrops. Patient Hospitalized 12/28/20 – current MFM consulting in hospital & outpatient | |
| COVID19 VACCINE (COVID19) | Redness about 1 1/2 inches around injection site, edema to right arm, r side of neck, r breast, r shoulder, temp 99.3, increased blood pressure. Patient went to primary care physician. She was then sent to the ER. She was Covid (+) in July 2020 | |
| COVID19 VACCINE (COVID19) | Shortly after receiving the vaccine (within 10 minutes) the patient’s tongue swelled, facial redness, gasping for air. This resident was marked for a 30 minute observation due to previous anaphylaxis type reaction. Immediately administered 0.3mg epinephrine x 1 dose. Then administered 50mg IM Diphenhydramine. This treatment course resolved the adverse reaction. Patient was monitored onsite at facility. Her husband came to pick her up and take her home. Tried to reach patient several hours after but was unable to at this time. | |
| COVID19 VACCINE (COVID19) | approximately 30 minutes after receiving vaccination i began to develop tongue and lip swelling as well as difficulty swallowing and breathing , i then proceeded immediately to the nearest er | |
| COVID19 VACCINE (COVID19) | Patient complained of a headache, then patient was losing consciousness and gasp for air. EpiPen was utilized due to being unresponsive then began to seizing. Patient started to flatline and an AED was used while a paramedic came. Patient was put on her side then facility gave something for seizures. Lastly, ,ambulance took her to the hospital. | |
| COVID19 VACCINE (COVID19) | Patient started having myalgia, chills, nausea on the next day of the vaccination. on 2nd day (12/29) patient had chest pressure which made her present to Hospital ED. She had troponin elevation to 1.14. Cardiac Catheterization was done which was negative. On Trans Thoracic Echocardiogram, patient was found to have hypokinesis of the mid and distal segment with some sparing of apex proving Takotsubo (stress induced) cardiomyopathy. Patient did not have any underlying emotional or physical stress going on in her life or family. Till now extensive infectious as well as inflammatory work up is done to rule out any secondary causes of cardiomyopathy which till date have remained negative. As a diagnosis of exclusion, her presentation seems to be COVID-19 vaccine induced Takotsubo Cardiomyopathy | |
| COVID19 VACCINE (COVID19) | Pt received 1st dose of Moderna vaccine in am at COVID vaccination clinic. On presentation to clinic he stated he was feeling well. Pt was brought to ER in pm with hypoxic, requiring 15L supplemental O2. Per pt’s family, pt was not feeling well for the last couple of days but didn’t think it was related to COVID. Abbott COVID + in ER. Possible vaccine reaction, though seems unlikely. | |
| COVID19 VACCINE (COVID19) | “15-20 mins after receiving the vaccine she reported she had difficulty swallowing and difficulty breathing and was ?shaking.”” a PA wrote in her note that when she ran in to help, she found the patient to be tachypneic, diaphoretic, warm with some red blotchy patches on face, chest & neck. Able to speak easily c/o trouble breathing & sensation of throat swelling & extremities feeling abnormal. No stridor. No facial edema noted by that clinician. Administered epi-pen 0.3mg – IV started , Benadryl 50mg IVP and solumedrol 125mg IVP. Patient reports she subsequently arched her back and had rigidity of her arms/legs and tremors. Clinic PA reports that while she was there, pt was never hypotensive. Initially hypertensive after epi as expected with some favorable response after 10-15 min Staff there gave her IM epinephrine, IV Solu-Medrol and 50 mg IV Benadryl. EMS was contacted and transported to the emergency room. She arrived at the ER, was monitored for 2 hours, was started on pepcid and benadryl and discharged from the ER. She had a diffuse itchy rash. The following day she again developed recurrence of throat swelling. Went back to a different ER. Developed dyspnea immediately prior to arrival at ER. There was again given solumedrol and benadryl and pepcid and developed muscle rigidity and arched back for 10 minutes. Symptoms of SOB and dyspnea resolved with epinephrine. Was discharged from the ER with prednisone after being monitored for 5 hours. Is continuing to take prednisone and benadryl. Rash is still present but improving with scheduled benadryl. Has new redness at injection site today. Continues to feel some throat swelling but no tightness today. This information was gathered from talking with pt today for a phone appt and also from her medical chart regarding her vaccination visit and two ER visits.” | |
| COVID19 VACCINE (COVID19) | Acute appendicitis, onset morning of 1/1/2021 (Reporting this because Pfizer covid vaccine had 3-4x higher risk of appendicitis, although data not reported for Moderna covid vaccine) | |
| COVID19 VACCINE (COVID19) | on dec 22 I felt some myalgias, chills, fatigue, HA –quite normal. That evening, noted small amount swelling R hand –I iced and took acetaminophen. By Dec 25, hand very swollen and painful with decreased ROM all fingers | |
| COVID19 VACCINE (COVID19) | Within 15 minutes of receiving the vaccine I began to get very itchy and blotchy with a hoarse voice. The paramedic downstairs walked me up to the emergency room. I was treated with medications to help calm the itching and burning feeling. By 940 I went anaphylactic and had several doses of epinephrine to help calm this. I continued to have rashes and the feeling of my throat closing. I was transferred by ambulance to medical center in the ICU. I am still here and have had two toner anaphylactic episodes since. I have been on a epi drip, steroids, famotidine, Ativan and Benadryl. I also had a picc like placed. | |
| COVID19 VACCINE (COVID19) | Anaphylaxis. Immediately experienced shortness of breath, rapid heart rate, and rash. I am a Nurse Practitioner in the emergency department. Had went down to the temporary vaccine station to receive my vaccine, immediately returned to the ER and began to experience symptoms of anaphylaxis. Was immediately placed in a treatment room and received treatment by the ER physician, which included oxygen, intravenous Benadryl, Solumedrol, and Normal Saline. Was observed for several hours and then eventually sent home with prescription for Prednisone and Pepcid. I do have a allergy to shellfish, was never asked about my allergies and nothing on the paperwork I was given prior to the injection noted a concern for shellfish allergies. | |
| COVID19 VACCINE (COVID19) | Flushing, sweating, increased heart rate proceeded to feel difficulty swallowing and clearing my throat. I was taken to the ER. The symptoms progressed to feeling dizziness, difficulty speaking, and chest pressure with increased SBP/DBP. General nausea and feeling very unwell. | |
| COVID19 VACCINE (COVID19) | CAREGIVER RECEIVED FIRST VACCINE DOSE AND SOON AFTER BEGAN TO FEEL DIZZY AND HER EYES BEGAN TO TWITCH, FOLLOWED BY UNCONTROLLED SHAKING, WITH HIGH FEVER FOLLOWED BY SEVERE SHORTNESS OF BREATH AND GASPING, WITH TIGHTNESS AROUND THE CHEST. TRANSPORTED TO EMERGENCY DEPARTMENT. HAD MULTIPLE EPISODES OF ITEMS LISTED ABOVE WHILE IN ED, INCLUDING HEAVINESS IN HER LEGS AND TINGLING IN ARMS. SHE WAS DISCHARGED FROM ED AT 10:30PM BUT WAS READMITTED ON 12/24 TO ED FOLLOWING SIMILAR ISSUES. TO DATE SHE HAS HAD 5 RAPID RESPONSES IN HOSPITAL DUE TO REPEAT OF SIGNS/SYMPTOMS. CT AND PULMONOLOGY CONSULT SCHEDULED FOR 12/28/2020. | |
| COVID19 VACCINE (COVID19) | Patient is a 55 year old male with no past medical history who presents with complaint of sudden onset of left-sided nonradiating chest discomfort of sudden onset approximately 1 hour prior to presentation while doing administrative work at rest. He describes the pain as a dull heaviness sensation and approximate 3/10 pain severity. Chest discomfort was associated with a feeling of flushing that was quite transient but chest discomfort was persistent. Patient immediately presented to the ED for further evaluation. He denies experiencing any chest pain upon waking up this morning. Does note that he did have a transient episode of epistaxis on his way to work for which he had to pull over and apply pressure to his nose but this subsequently subsided and he attributed this to dry air as he has experienced epistaxis in the past but with less severity previously. In the ER, vital signs noted for BP 133/76, pulse ranging 91-114, respiratory rate 16-20, 96% on room air. Initial laboratory parameters were completely normal including normal CBC, CMP, LFT, lipase, UA, and normal D-dimer. Initial troponin was negative x1. EKG with sinus tachycardia, heart rate of 115. Noted Q waves inferiorly. No acute ST or T wave changes appreciated. Chest x-ray with mild increased density in the left lower lobe. Given this, patient was tested for rapid Covid which was negative but PCR was positive for COVID. CT of the chest noted for focal subsegmental groundglass infiltrate at the superior segment of the LLL, likely infectious versus inflammatory. Also noted small nonspecific groundglass attenuation with focal septal thickening at the right upper lobe which could be infectious or inflammatory, bibasilar atelectasis. Patient was treated with aspirin 324 mg in the ED. Of note, patient actually just received the COVID-19 vaccination on 12/24/20. He denies any shortness of breath, no cough, denies any nausea or vomiting, denies any change in taste or smell nor change in appetite. Does note 1 single episode of loose stool but otherwise denies any diarrhea. Does report that he had approximate 48 to 72-hour period of fatigue and soreness at the site of the left deltoid injection following the vaccination but otherwise no further symptoms. It is also noted that he does have a positive family history of coronary artery disease as his dad had an MI at the age of 49. Patient has never undergone a cardiac catheterization in the past but does report having a negative stress test at the age of 42. He is being admitted under the hospitalist service for further management Patient was initially admitted under observation for chest pain obs. However patient’s Covid test came back positive and patient also had dynamic EKG changes concerning for possible unstable angina. Patient was treated with aspirin Plavix full-strength Lovenox along with beta-blocker and a cardiology consult. Serial troponins were negative. Echocardiogram revealed normal EF of 55 to 60% with no hemodynamically significant valvular disease. Cardiology felt that patient likely has underlying coronary artery disease have recommended discharge home with aspirin and Plavix with outpatient stress testing given his positive Covid testing. At the time of discharge patient denied any chest pain or shortness of breath. Patient was borderline diabetic with a hemoglobin A1c of 6.1. Patient was discharged home with Metformin along with glucometer, glucose strip, lancets. Given patient’s tachycardia patient’s Metformin 25 mg twice daily was changed to Toprol 25 mg daily. (Please note clarification in comparison to discharge home med list. Toprol XL 25 mg daily was called to pharmacy in place of the metoprolol.) | |
| COVID19 VACCINE (COVID19) | The vaccine was received at 1:12 PM, and I felt fairly fine, aside from injection site pain and some tingling in my left arm until I had sudden significant elevation of heart rate, with shortness of breath, and throat swelling/tightening at approximately 1:26PM. I cold compress was applied to my forehead and I was put in a reclining position & then received Epinephrine at 1:28PM. EMS (present onsite) arrived for transport at 1:31PM. 4L of oxygen was applied after O2 sat of 89% noted by EMS. Blood pressure was elevated to >200/100 initially by EMS. Symptoms improved quickly following epinephrine, with some residual feelings of very mild throat fullness, and I developed chills which improved over time. I was transported to emergency department where I was evaluated (symptoms mostly resolved at that time, but ED physician noted a little swelling remaining in my uvula), then IV Benadryl and Decadron were given. Later acetaminophen was also given for headache that developed during my ED stay. My vitals were monitored throughout and observation occurred until I was discharged at approximately 5:00PM, as symptoms had not recurred. | |
| COVID19 VACCINE (COVID19) | HIVES, SOB, THROAT CLOSING UP, WHEEZING | |
| COVID19 VACCINE (COVID19) | 12/28/2020, Pharmacy staff administered Moderna COVID Vaccine. 12/29/2020, he had not eaten breakfast or lunch but did consume fluids and take his medications. BP =150/70, Temp. = 101.6, Pulse= 102, Respirations= 18 and Oxygen saturation= 97%. Tylenol 650 mg given. It was difficult for him to swallow. Also had no use of right upper extremity and unable to move lower extremity, mouth was drooping and was drooling. Physician in attendance and ordered to send to ER. 1/1/2021, received information from nurse at hospital that patient received a Peg Tube this afternoon and Clinical indication of a stroke. | |
| COVID19 VACCINE (COVID19) | I received the vaccine at 6:30pm on 12/27. I worked atb7pm and took lunch at 2:00am. After lunch, I immediately felt sick to my stomache and threw up. I went home after my shift and went to bed. After 5 hours, I woke up and went to the couch to lay down while kids watched TV. Next, I woke to several people in my house. I had a seizure and my son had called 911. I was taken to emergency department. I was admitted and stayed 2 nights in the hospital. | |
| COVID19 VACCINE (COVID19) | After vaccination, patient tested positive for COVID-19. Patient was very ill and had numerous chronic health issues prior to vaccination. Facility had a number of patients who had already tested positive for COVID-19. Vaccination continued in an effort to prevent this patient from contracting the virus or to mitigate his risk. This was unsuccessful and patient died. | |
| COVID19 VACCINE (COVID19) | A little over ab hour after receiving the vaccine I noticed a burning sensation in my sinuses. By 130am 1/1/2021 I awoke from my sleep terribly dizzy, shaking violently and experiencing a fever of 101.3 F. I took advil and tylenol and fell asleep about an hour later. I woke up with similar symptoms at approximately 830am on 1/1/2021 took an additional dose of advil and tylenol and slept till 12p. I woke up with a bad headache and coughing fits similar to when I had covid back in March. I went to an urgent care who assessed me and ordered me to the ER. medical center administered IV fluids, an inhaler, steroids, epinephrine and benadryl and a few hours later my symptoms had subsided for the most part and a dose of IV antibiotics was administered. I am currently admitted for observation with likely discharge on 1/2/2021. | |
| COVID19 VACCINE (COVID19) | 30YO F ICU nurse obesity (BMI 35) COVID 19 on Dec 2 symptoms, Dec 3 tested positive for COVID-19. never hospitalized, outpatient only. 12/12 completed isolation 12/21 received vaccine 12/7 developed Fever chills diarrhea SOB cough Urgent care visit. RLL consolidation on CXR given doxycycline 100 mg po bid worse, fever 40 targetoid lesions to LE (started before doxy) WBC 22K tachycardic tachypneic admitted requiring 2-4L oxygen CT angio without clot, diffuse ground glass and RML dense infiltrate DDimer 7.8 LDH 599 CRP 41 procal 0.67 ferritin 500 Viral respiratory PCR negative Sputum cx with oral flora (pending) COVID ag testing neg COVID PCR 1/3 targets positive (called as indeterminate). | |
| COVID19 VACCINE (COVID19) | Within 5 minutes of the vaccine, patient had wheezing, shortness of breath and chest pain. patient given epi x 4, decadron, fluids with some improvement and then hospitalized. IN the hospital, patient continued to have chest pain and satting well but with protracted course and is still in the hospital. | |
| COVID19 VACCINE (COVID19) | Vaccine given at 7:05am 12:00noon, 5 hours later, I started experiencing severe chest pain, jaw pain and shortness of breath in which EMS was called and I was taken to the hospital. Since then, I lost feeling in my hands and feet, numbness and tingling. I’ve improved however, during my recovery suffered with spinal pain, shortness of breath, very winded, muscle pain and loss of appetite to especially meat. | |
| COVID19 VACCINE (COVID19) | Soreness and weakness of left arm for more than 5 days. Intermittent headaches and runny nose | |
| COVID19 VACCINE (COVID19) | On 12/29: lightheadedness, flushed, felt like I was going to pass out, numbness/tingling down arms and hands, chest tightness, clammy hands and feet. | |
| COVID19 VACCINE (COVID19) | Anaphylaxis. The COVID shot was given, no reaction then. After 7 minutes, congestion, severe cough, vomiting phlegm, feeling like throat closing started happening. Code was called, Benadryl was immediately given intramuscular in the left arm, blood pressure, pulse ox was taken, and then was taken to the Emergency Department. In the ED, I was given prednisone, one EPI, anti-nausea medication all through I.V. and many more medications given to me via I.V. that I don’t sincerely remember. I was under observation for 4 hours. I was discharged after all symptoms dissipated and was given Prednisone 20 MG (3 tabs a day) to take to help my lungs. Management followed up almost immediately, everyone from the moment I had the anaphylactic reaction was quick and prepared. | |
| COVID19 VACCINE (COVID19) | Patient with a history of thalassemia and Gilbert’s disease, developed severe jaundice three days after vaccination. Had mild headache and sore arm but otherwise felt well. Had labs drawn – found to have highly elevated bilirubin (23) and LFTs in the 700s. Was admitted to the hospital and had CT showing Cholelithiasis, choledocholithiasis and minimal intrahepatic biliary ductal dilatation. Left hospital and was admitted to another facility where plan was for ERCP and cholecystectomy. Ultimately unclear if at all related to the vaccination – may be coincidental. | |
| COVID19 VACCINE (COVID19) | Pt had vaccination at city site. Waitied 15 min after shot and was cleared to go. Reported to wife that he was very thristy, so they stopped at a convenience store on the way home. While there, he felt worse and asked to go to the Emergency room. They chose Methodist to enter. Pt went to triage and while at triage, had syncopal episode, then full arrest. After short course of CPR and defib, he had ROSC. Was taken to cath lab for intervention (stents) and is now in ICU. | |
| COVID19 VACCINE (COVID19) | At the time of vaccination, there was an outbreak of residents who had already tested positive for COVID 19 at the nursing home where patient was a resident. About a week later, patient tested positive for COVID 19. She had a number of chronic, underlying health conditions. The vaccine did not have enough time to prevent COVID 19. There is no evidence that the vaccination caused patient’s death. It simply didn’t have time to save her life. | |
| COVID19 VACCINE (COVID19) | Prior to the administration of the COVID 19 vaccine, the nursing home had an outbreak of COVID-19. Patient was vaccinated and about a week later she tested positive for COVID-19. She had underlying thyroid and diabetes disease. She died as a result of COVID-19 and her underlying health conditions and not as a result of the vaccine. | |
| COVID19 VACCINE (COVID19) | Tactile fever ,arm pain, headache and malaise in 24 hrs following injection Next day generalized achiness ,retrosternal chest pain and bilateral forearm tingly pain similar to Nov 2019 and went to Hospital UC,CXR and EKG normal but with short PR interval on EKG ,elevated troponin 3.5 Transferred to hospital troponin 12.1 ng/ml IVIG given SARS IGG positive on admission PCR negative | |
| COVID19 VACCINE (COVID19) | ON 1/1/21 THE DAY AFTER I RECEIVED THE VACCINE I WAS TAKING A NAP AND MY WATCH KEPT SENDING ME ALERTS. HOWEVER I DID NOT CHECK MY WATCH UNTIL 5:30 WHEN I WOKE UP AND I FELT LIKE I WAS HAVING HEART PALPITATIONS. I WENT TO THE EMERGENCY ROOM WHERE I WAS TREATED. I WAS TOLD THAT I WAS FEBRILE WITH A TEMPERATURE OF 102. I WAS GIVEN IV FLUIDS, CHEST X-RAY, EKG AND LAB WORK. I WAS RELEASED ON 1/2/21 AT APPROXIMATELY 0030 (MIDNIGHT). | |
| COVID19 VACCINE (COVID19) | “Patient is hospital employee who completed screening form for COVID-19 vaccine by answering “”no”” to all contraindication questions. Approx 10 minutes after receiving COVID-19 vaccine dose # 1, patient was still in vaccine clinic area and complained of dizziness, palpitations and flushing. I observed patient fanning herself with papers. She was escorted out of the immediate clinic room, and assessed by paramedics present as having an anaphylactic reaction. Epinephrine 0.3 mg IM and diphenhydramine 50 mg IV given in clinic, Rapid Response was called overhead and patient immediately transported down the hall to the Emergency Dept. In ED, pt was noted as having swollen tongue, large areas of erythema on face, arms and chest, shortness of breath, nausea, dizziness (per ED physician notes). Pt reported being hospitalized in ICU with COVID disease more than 3 months ago, including intubation (not treated at this hospital), and has been back at work since August 2020. ED physical exam noted bilateral wheezing and patient in acute distress. In ED, pt administered racemic epinephrine 2.25% 0.5 mL via neb, epinephrine 0.3 mg IM, diphenhydramine 50 mg IV, Solu-Medrol 125 mg IV, famotidine 20 mg IV and epinephrine 5 mg/250 mL IV drip (started at 0.118 mcg/kg/min). Acute symptoms reported to resolve in ED. COVID test was negative. ED physician discovered that pt had history of multiple medications, including previous anaphylactic reaction to radiocontrast dye requiring intubation (which was not disclosed on the vaccine screening form). Pt admitted to Telemetry floor for observation. Overnight course was unremarkable, and patient was discharged the following day with prescription for Prednisone taper and prescription for Epi-pen. Advised not to return for second dose of COVID vaccine. EMR updated to reflect possible anaphylactic reaction to Moderna COVID-19 vaccine.” | |
| COVID19 VACCINE (COVID19) | I suffer from lingering SOB with exertion after COVID infection. On the night of 12/31/2020 I began to feel more SOB than usual and was unable to correct my SOB with rescue inhaler. Became more SOB with exacerbated tachycardia and tachypnea, My family had to call 911 because I became aphasic and showing signs of possible stroke. I was taken to the ER and admitted for respiratory recovery and to role out stroke. The stroke was ruled out and I recovered with IV prednisone therapy, twice daily and supplemental oxygen. Released after HR, BP, & respiratory effort returned to normal: 01/03/2021 | |
| COVID19 VACCINE (COVID19) | I was 28 weeks and 5 days pregnant when I received the first dose of the COVID19 vaccine. Two days later (12/25/2020 in the afternoon), I noticed decreased motion of the baby. The baby was found to not have a heartbeat in the early am on 12/26/2020 and I delivered a 2lb 7oz nonviable female fetus at 29 weeks gestation. I was 35 years old at the time of the fetal demise and the only pregnancy history for this pregnancy included a velamentous cord insertion that was being closely monitored by a high risk OB. My estimated due was March 12, 2021. | |
| COVID19 VACCINE (COVID19) | At around 40 hours post vaccination, developed severe abdominal pain and went to an emergency room for evaluation on 1/1/21. Abdominal pain was eventually diagnosed as appendicitis requiring appendectomy on 1/2/21. Emergency room visit and hospital discharged patient early on 1/2/21. It was then determined that the on-call team covering mis-read the CT scan and acute appendicitis was found. Patient then went to Medical Center on 1/2/21 for appendectomy and was discharged later that night following operation. | |
| COVID19 VACCINE (COVID19) | 1/1/2020: Residents was found unresponsive. Pronounced deceased at 6:02pm | |
| COVID19 VACCINE (COVID19) | Hospitalized with COVID-related pneumonia on 03 Jan 2021. Close contact exposure on 25 Dec, with positive COVID PCR test on 29 Dec… managed as outpatient until respiratory sxms prompted hospitalization on 03 Jan. Care team anticipates at least 4 inpatient days… but patient remains hospitalized at date of this report. | |
| COVID19 VACCINE (COVID19) | Patient developed SVT 15 minutes after receiving vaccine. Admitted to ICU. ER presentation: BP: 160/109 heart rate 132. No e/o anaphylaxis or allergic reaction. | |
| COVID19 VACCINE (COVID19) | Pfizer-BioNTech COVID-19 Vaccine EUA – Patient witnessed another patient with syncope prior to her injection. She was already anxious about receiving vaccination and this increased her anxiety, though she proceeded with immunization. Patient was in 15 min observation window in a chair and began to feel light-headed like she may pass out. A SWAT was called. With RN assistance, patient was lowered to the floor, with no loss of consciousness. Patient was pale and reported anxiety, racing /pounding heart, and felt hot with facial flushing. Patient was transferred to ED and was noted to be tachycardic (120s), but dropped to 80s. She noted that this episode felt different than her prior syncopal episodes associated with anemia. Patient was observed for 5 hours and discharged to home. Patient returned to ED roughly 2.5 hours later complaining of continued dizziness and unsteady gate. Patient was pale and anxious. Patient reported had not eaten/drank enough during her shift and received vaccine immediately post a stressful shift. Additionally, patient witnessed another patient have syncopal episode prior to her receiving her vaccine which made her anxious. Patient was given IV fluids and had electrolytes replacement. Patient additionally received diazepam. Patient was discharged at 2358 on 12/23. Patient returned to ED on 12/24 at 0239 complaining of near syncope and lightheadedness. Patient had tachycardia and self-reported palpitations. Received IV fluids and observation on telemetry with no rhythm disturbance. Patient discharged 1428 on 12/24. On 12/29, patient returned to ED at 0326 for continued dizziness, fatigue and near syncope. Was admitted for cardiac evaluation. Noted to have unprovoked tachycardia and was discharged with a Halter Monitor to evaluate cardiac symptoms. patient was discharged 12/31 at 1619 | |
| COVID19 VACCINE (COVID19) | “Pfizer-BioNTech COVID-19 Vaccine EUA – Patient with history of anaphylaxis requiring intubation to benzonatate. Patient answered “”no”” to questionnaire about allergic reactions prior to vaccination. 11 minutes after vaccination, patient reported tingling of lips and swelling of face. Developed hoarseness. SWAT was called and patient given benadryl and taken to ED (1055). Patient received steroids and H1/H2 blockers in addition to epinephrine. Patient brought to ICU for monitoring. Patient continued on therapy and was discharged 1/2 at 1113. Patient returned to ED on 1/3 at 1558 with macular papular rash on leg, chest and back with itching on eyelids and face. No respiratory involvement. Patient given benadryl and predinisone and discharged from ED at 2016.” | |
| COVID19 VACCINE (COVID19) | About 6 hours after receiving dose, experienced aching in both arms that extended down into both hands – hands was resolved by next morning. Achiness in both arms remained x 48 hours | |
| COVID19 VACCINE (COVID19) | Resident found unresponsive without pulse, respirations at 04:30 CPR performed, expired at 04:52 by Rescue | |
| COVID19 VACCINE (COVID19) | Patient complained of increased shortness of breath, generalized weakness and fatigue with mild cough worsening today and was admitted on 12/25/20. Patient is an employee of the hospital in the ICU and received the covid-19 vaccine on 12/25/20. Patient believes symptoms started after the vaccination. On admission, patient was in sinus tachycardia with O2 saturation 91% on room air. Tested SARs CoV 2 RNA, RT PCR positive on 12/24/20. Transferred to ICU for closer monitoring after transitioning to high flow nasal cannula on 12/26. Patient recovered and discharged on 12/31/20. | |
| COVID19 VACCINE (COVID19) | Resident became SOB, congested and hypoxic requiring oxygen, respiratory treatments and suctioning. Stabilized after treatment and for the next 72 hours with oxygen saturations in the 90s. On 1/3/2021 was found without pulse and respirations. Resident was a DNR on Hospice. | |
| COVID19 VACCINE (COVID19) | Two days post vaccine patient went into cardiac arrest and passed away. | |
| COVID19 VACCINE (COVID19) | syncopal episode – arrested – CPR – death | |
| COVID19 VACCINE (COVID19) | Severe diarrhea, cold chills, 101 fever, and nausea | |
| COVID19 VACCINE (COVID19) | “””Pfizer-BioNTech COVID-19 Vaccine”” 12/29 patient developed SOB, fever tmax 103 degrees F, diaphoretic, dry heaves all started approximately 16 hours after vaccination given. patient then transferred to Hospital for further treatment and observation. 12/30 seen at injection site- erythema, swelling, warmth and tenderness Discharged back to home on 1/1 with RX for cephalexin to treat cellulitis of injection site” | |
| COVID19 VACCINE (COVID19) | cardiac arrest; heart failure; did not feel well, lost consciousness and died; did not feel well, lost consciousness and died; This is a spontaneous report from a contactable consumer. A 75-year-old male patient received bnt162b2 (PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration on 28Dec2020 08:30 at single dose for covid-19 immunisation. Medical history included suffered from the past from heart attacks, active heart disease, malignant disease. The patient’s concomitant medications were not reported. A man of 75 years old, who suffers from many different background diseases, died (this morning 28Dec2020) from cardiac arrest, two hours after he received the injection. The man received the injection at 8.30am, and after he was feeling okay he was released to go home. After a while when he was home he did not feel well, lost consciousness and died, and he was pronounced dead from heart failure. The patient died on 28Dec2020. It was not reported if an autopsy was performed. The outcome of the event cardiac arrest and heart failure was fatal while the outcome of the other events was unknown. No follow-up attempts are possible; information about lot/batch number cannot be obtained.; Sender’s Comments: Linked Report(s) : IL-PFIZER INC-2020517177 same reporter, same vaccine, reporting similar events in different patients.; Reported Cause(s) of Death: heart failure; cardiac arrest | |
| COVID19 VACCINE (COVID19) | found dead in his bed; This is a spontaneous report from a contactable healthcare professional received via the Ministry of Health department of epidemiology. The department of epidemiology reported similar events for two patients. This is the second of two reports. A 61-year-old male patient received the first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 mRNA VACCINE; Lot Number: EK4175), via an unspecified route of administration on 24Dec2020 as a single dose for COVID-19 immunization. Medical history included schizophrenia, very heavy smoker for almost 50 years, emphysema, and tumor resection in the bladder. The patient’s concomitant medications were not reported. On 28Dec2020, the patient was found dead in his bed. It was reported that the patient did not have any complaints in the days following the vaccination. Then, on 28Dec2020, the patient was found dead. The cause of death was unknown. It was not reported if an autopsy was performed.; Sender’s Comments: A reasonable possibility that the event unknown cause of death is related to vaccination with BNT162B2 cannot be completely excluded until further information regarding clinical course and death cause is provided. Of note, the patient did not have any complaints in the days following the vaccination. The case was confounded by the patient’s underlying conditions. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate.,Linked Report(s) : IL-PFIZER INC-2020517122 same reporter, same vaccine, reporting similar events in different patients.; Reported Cause(s) of Death: found dead in his bed | |
| COVID19 VACCINE (COVID19) | died the day after receiving the first injection of vaccine against Covid-19 in suspected cardiac arrest; This is a spontaneous report from a web page with a contactable physician as publisher. A multi-sick, elderly patient of an unspecified gender received the first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration on an unspecified date at single dose for covid vaccination. The patient medical history was not reported. The patient’s concomitant medications were not reported. The patient died the day after vaccination of a suspected heart stop. The patient died the day after receiving the first injection of vaccine against covid-19. The patient died on an unspecified date. It was not reported if an autopsy was performed. No follow-up attempts are possible; information about LOT/batch number cannot be obtained.; Sender’s Comments: The information available in this report is limited and does not allow a medically meaningful assessment of the case. In particular the following relevant information is not available: complete medical history and complete demographics, treatment dates and dose, concomitant medications (if any), event descriptors, autopsy report. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to regulatory authorities, Ethics Committees, and Investigators, as appropriate.; Reported Cause(s) of Death: suspected heart stop | |
| COVID19 VACCINE (COVID19) | Scratchy throat; itching lips; This is a spontaneous report from a non-contactable nurse. A 36-year-old patient of an unspecified gender received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE), lot number EK5730, via an unspecified route of administration on Right arm from 22Dec2020 to 22Dec2020 as single dose for COVID-19 immunization. Medical history included food allergy (Shrimp). The patient’s concomitant medications were not reported. The patient experienced scratchy throat and itching lips for approximately 1.5 hours starting 20 mins post vaccine on 22Dec2020. The event caused prolonged hospitalization. The outcome of the events was recovered on 22Dec2020. No follow-up attempts are possible. No further information is expected.; Sender’s Comments: Based on temporal association, a possible contributory role of suspect BNT162B2 vaccine cannot be excluded for reported events throat irritation and lip itching. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | delirious; hypoxic; fever; ataxic; incontinent; confused; Chills; HA; anorexia/had no appetite; myalgias; extreme fatigue; slept all and had no appetite; This is a spontaneous report from a contactable physician. A 74-year-old male patient received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration on 15Dec2020 16:00 at single dose for COVID-19 immunization. Medical history included diabetes mellitus (DM). The patient has no known allergies. The patient’s concomitant medications were not reported. He was an ER doctor and the medical director of his hospital. The patient was asymptomatic when he got the vaccine on 15Dec2020. 3 hours after the vaccine he began to get chills, HA, anorexia, myalgias, and extreme fatigue. This worsened and he slept all and had no appetite. On 19Dec2020 he woke up delirious with a fever and was ataxic, hypoxic, incontinent, and confused. The patient was hospitalized due to the events on 15Dec2020. The events also caused prolonged hospitalization due to the events. The patient was not diagnosed with COVID prior to vaccination. The patient was tested for COVID via nasal swab post vaccination with unknown results. The patient did not receive any other vaccines within 4 weeks prior to COVID vaccine. The outcome of the events was not recovered. Therapeutic measures were taken as a result of the events as the patient required oxygen, plasma, and remdisivir. The batch/lot numbers for the vaccine, BNT162B2, were not provided and will be requested during follow up.; Sender’s Comments: A possible causal association between administration of BNT162B2 and the onset of reported serious events might not be excluded, considering the plausible temporal relationship. Fever, chills, headache, fatigue and muscle pain are the known adverse event profile of the suspect product. More information such as detailed underlying medical conditions and concomitant medications are needed for fully medical assessment. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | Small bowel obstruction with diffuse bowel and pelvic lymphadenopathy 36 hours after injection; Small bowel obstruction with diffuse bowel and pelvic lymphadenopathy 36 hours after injection; This is a spontaneous report from a contactable physician (patient). A 61-years-old female patient (not pregnant) received the first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE, Lot number: EH9899), intramuscularly on 21Dec2020 12:30 to at single dose on left arm for COVID-19 immunization in hospital. Medical history included crohn’s disease. No known allergies. Concomitant medications within 2 weeks of vaccination included estradiol, progesterone, colestipol hydrochloride (COLESTID), ustekinumab (STELARA), cyanocobalamin (B12). The patient did not receive any other vaccines within 4 weeks prior to the COVID vaccine. The patient experienced small bowel obstruction with diffuse bowel and pelvic lymphadenopathy 36 hours after injection on 22Dec2020 22:00 with outcome of recovered in Dec2020. The adverse events resulted in emergency room/department or urgent care, hospitalization for 3 days. Therapeutic measures were taken as a result of event included inpatient observation, nothing by mouth (reported as NPO), intravenous fluids. No COVID prior vaccination, COVID test nasal swab was negative on 23Dec2020 post vaccination. It was not reported as serious.; Sender’s Comments: There is not a reasonable possibility that reported events small bowel obstruction and lymphadenopathy are related to BNT162B2 vaccine. The patient had underlying Crohn’s disease, which put patient at risk of developing the event. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | She was hospitalized for a month Occurred after a flu two months prior and a stomach flu 2-3 weeks prior; She was hospitalized for a month Occurred after a flu two months prior and a stomach flu 2-3 weeks prior; This is a spontaneous report from a contactable consumer. A 75-year-old female patient received bnt162b2 (BNT162B2, lot number and expiry date were not reported), via an unspecified route of administration on an unspecified date at a single dose for covid-19 vaccination. Medical history included guillain-barre syndrome from 2011 at the age of 65 and Levaquin allergy. The patient’s concomitant medications were not reported. On an unspecified date, the patient was hospitalized for a month that occurred after a flu two months prior and a stomach flu 2-3 weeks prior. The outcome of the events was unknown. Information about lot/batch number has been requested. | |
| COVID19 VACCINE (COVID19) | Difficulty breathing; This is a spontaneous report from a contactable pharmacist. A 54-year-old female patient received her first dose of intramuscular BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE) on 22Dec2020 at 04:15 PM at single dose in left arm for COVID-19 immunisation at the age of 54-year-old. Lot number was ELO140. Medical history was unknown, concomitant medications were unspecified. Patient was not pregnant at the time of vaccination. On 22Dec2020 at 04:30 PM, the patient experienced difficulty in breathing, and she was hospitalized for one day. The patient was treated with EPI for the event. The patient was recovering from the event.; Sender’s Comments: Based on the compatible temporal association, the Company considers the event difficulty in breathing is possibly related to vaccination with BNT162B2. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | abdominal pain; nausea; high blood pressure; This is a spontaneous report from a contactable consumer. A 93-year-old male patient received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE, lot number: EL0140), via an unspecified route of administration on 22Dec2020 at a single dose for COVID-19 immunization. Medical history included diabetic and irritable bowel. The patient’s concomitant medications were not reported. The patient received the COVID vaccine and had abdominal pain, nausea and high blood pressure within 12 to 18 hours of vaccine received. The events lead to nursing home to emergency room and admitted to hospital. The patient was hospitalized due to events since 23Dec2020. Outcome of the events was recovering. | |
| COVID19 VACCINE (COVID19) | “left sided weakness; it has weakened his heart; stutter; severe stroke like symptoms; Ventricular tachycardia/help keep his heart rate at bay; Loss of balance; extreme numbness and tingling in left hand and foot; tingling in left hand and foot; oral motor impairment; mouth weakness and not coordinated/mouth is fatigued easily; Issues finding words and trouble speaking; Issues finding words and trouble speaking; Ejection fraction down to 25%; The initial case was missing the following minimum criteria: adverse event. Upon receipt of follow-up information on 28Dec2020, this case now contains all required information to be considered valid. This is a spontaneous report from a contactable other healthcare professional (HCP). A 29-years-old male patient received bnt162b2 (lot number: EJ1685), intramuscular (deltoid left) on 21Dec2020 at 05:30 at 0.3 mL single (first dose) for Covid. Medical history was reported as “”none””. Concomitant medications were not reported. The patient previously received Flu vaccine in Oct2020 for immunization. The patient is an Occupational Therapist, and he called to report an adverse event that he experienced with the first dose of the COVID Vaccine. He received the vaccine last Monday, 21Dec2020 at 5:30AM before his shift at work, then 20 minutes later, he was having severe stroke like symptoms. He experienced severe left sided weakness, loss of balance, extreme numbness and tingling in his left hand and foot, he had issues finding his words and he couldn’t speak, and he had an oral motor impairment where his mouth was weak and not coordinated. The staff at the hospital did a neurological exam on him, and he failed, so he had to go to the emergency room (ER). The patient added that he was already in the hospital when this happened, and the ER doctors suspected that he had a CVA, and they gave him TPA to prevent any permanent brain damage and it worked. The patient then added that due to the shock of this whole event, from everything that happened, it has weakened his heart. Reportedly, he is a healthy 29 year old man, with no preexisting conditions, and he works out, and he has no heart conditions, but he had to get a cardiology follow up a few days after he got the vaccine, because he started going in to Ventricular Tachycardia, which he had never had in his life. So, the doctors at the hospital went ahead and did an Echocardiogram and an EKG, and he was told that his Ejection Fraction is down to 25%. He stated his heart is so weak, that he cannot work right now, but the structure of his heart is fine and has not had any damage. The hospital staff thought that maybe the patient had a chronic heart issue that he just did not know about, and that the stress of this event maybe made it kick into overdrive, but he states that the cardiologist said that was not the case, because the structure of his heart is fine, and the only thing they can see is that the heart is pumping weak. One physician even suggested that due to the shock of the event, he might have Takotsubo Cardiomyopathy, which is a broken heart, but because the structure of his heart is okay, it should be reversible. He stated that he is hoping he will heal up good, because he is young and has no pre-existing conditions. He added that his heart is in such a state right now; he has to wear an external defibrillator. The patient stated that all these happened about 20 minutes after he received the vaccine, and he was admitted to the hospital from 21Dec2020 to 25Dec2020. His neurological symptoms have resolved except that he has a stutter that he did not have before and his mouth is fatigued easily, so he has to slow down when he is eating, but now he can eat regular for the most part. The patient confirmed that he was not specifically prescribed the product; it was administered to him at his place of work, but it was optional. He stated he considered how he is working with COVID patients every day, and given the circumstance, he thought that it would be a best practice for him to get the vaccine. He had not gone to his primary care doctor in a while because he had been fine and healthy, but he called them and found out that his primary care had retired, so he has to find a new one now. Regarding the issues finding words and trouble speaking, he stated that he has improved, but it is still ongoing, he is just stuck in a plateau zone. With the Ventricular Tachycardia, he stated that this is an ongoing issue, as he has to wear the life vest even though he has no need to activate it yet. He did have one minor bought of the VTach, but because he is a therapist, he knows how to take care of it with relaxation techniques, he knows how to manage it. He had one bought of VTach the evening prior, but he was able to get it under control. The doctors have him on medication to help keep his heart rate at bay. He has never had to use medication before and is on the following medications to help keep his heart rate at bay: Metoprolol 25mg one tablet once daily by mouth and Lisinopril 5mg one tablet once daily by mouth. The VTach has improved, it was good enough he was able to discharge home, but it is still a concern. His cardiologist said that, basically his hope, is that once his body recover from the whole shock of everything, then his ejection fraction will heal, and his heart will heal. He again stated that the doctor told him that the structure of his heart is perfectly fine; he has thick walls in his heart, no leaking valves, and the heart was not conducting any abnormal signals. The doctor just said that right now, his heart is super weak and that it is an acute problem. With the Takotsubo Cardiomyopathy, he states that two doctors mentioned this diagnosis, but he confirmed that he was not actually diagnosed with this issue, he was just diagnosed with Ventricular Tachycardia. The outcome of the ejection fraction down to 25% was unknown to the patient at this time as he has not had another EKG or echocardiogram, but the cardiologist told him that the cardiologist expects that this will not be resolved quickly anyway. The patient confirmed that he did not receive any other vaccines on the same day he received the COVID vaccine. The only other vaccine he had this year was the flu vaccine which he got back in Oct2020. He has gone on to his online portal and there are the bloodwork results and all the imaging results on there from his CTs and MRIs, but he did not see the EKG or Echocardiogram results yet. He does not have this pulled up at this time, but he does have access to this stuff and can provide it later, if requested. He is curious about the next steps from here to how his case is processed. He is also curious if this information would help Pfizer make modifications to the vaccine if it is found that a lot of people are having the same reaction as he did. He is also wondering, given his situation, that probably he is not going to get the second dose, for his safety, but he is wondering what percentage of effectiveness the first dose does having just covered. The events left sided weakness, loss of balance, extreme numbness and tingling in left hand and foot resolved on 25Dec2020; severe stroke like symptoms and oral motor impairment; mouth weakness and not coordinated/mouth is fatigued easily resolved in 2020. The events ventricular tachycardia/help keep his heart rate at bay and issues finding words and trouble speaking were resolving, stutter had not resolved while the outcome of the events it has weakened his heart, and ejection fraction down to 25% was unknown.; Sender’s Comments: The reported information is unclear and does not allowa meaningful assessment of the case. It will be reassessed upon receipt of follow up information. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to regulatory authorities, Ethics Committees, and Investigators, as appropriate.” | |
| COVID19 VACCINE (COVID19) | She had an immediate reaction of accelerated heart rate and elevated blood pressure; She has very slightly elevated heart enzymes.; She had an immediate reaction of accelerated heart rate and elevated blood pressure; She’s very anxious and very anxious tonight being alone at the hospital.; This is a spontaneous report from a contactable consumer (patient’s father). A 30-years-old female patient received the first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration on 18Dec2020 at single dose for COVID-19 immunisation. The patient medical history and concomitant medications were not reported. The patient had an immediate reaction of accelerated heart rate and elevated blood pressure. She was monitored for an hour and it subsided. On unspecified date in Dec2020 at 1:00am in the morning she had racing heart rate and went to ER. She was still in hospital. Things were not entirely stabilized. She had very slightly elevated heart enzymes in Dec2020 with outcome of unknown. They keep her overnight for an echocardiogram in morning. The patient was very anxious being alone at the hospital. Caller questioned if this has been reported with the vaccine. Lot/Batch and Expiry date has been requested. | |
| COVID19 VACCINE (COVID19) | Gallbladder removed, septic, 11mm axillary lymph node. | |
| COVID19 VACCINE (COVID19) | Approximately 4 days after vaccine I started experiencing sharp lower back and left hip pain. Also my left foot feels like pins and needles. | |
| COVID19 VACCINE (COVID19) | Complete loss of vision in the left eye 12 hours after receiving second dose (Moderna mRNA-1273) while having a fever of 102 F for 6 hours. Loss of vision lasted for 1 minute. Loss of vision occurred while standing. Referral to primary care and ophthalmology specialist found normal eye exam and MRI of orbits but presence of tachycardia especially while standing (fluctuations between 60 beats at rest/laying down to 130 beats per minute standing). Postural tachycardia syndrome (POTS) is suspected. Currently pursuing cardiac workup with cardiologist and Covid POTS specialist. POTS specialist believes autoantibody development after vaccination could be suspected as recovering covid patients similarly present to clinic with POTS like symptoms. | |
| COVID19 VACCINE (COVID19) | Patient with extreme nausea and vomiting that started soon after receiving the Moderna vaccine. Patient with loss of consciousness, diaphoresis and garbled speech during a foley catheter exchange thought to be from dehydration. Patient was admitted to Hospital for observation for 2 days | |
| COVID19 VACCINE (COVID19) | 12/18 VACCINATION 12/19 WOKE UP, RINGING IN BOTH EARS. CALLED PCP, CONSULTED ENT ABNORMALITY – L INNER EAR; HIGH DOSE STEROIDS, 10 DAYS, 60 MG/DAY. WEEK 2; TINNITUS GOT WORSE. DR. PRIMARY CARE PHYSICIAN MEDICAL EXAM ON 1/6/2021 INJECTION OF STEROIDS. | |
| COVID19 VACCINE (COVID19) | Acute Pericarditis. Patient was admitted from 12/27-12/28/2020 at hospital by cardiology team who strongly felt the acute pericarditis was due to the Pfizer Vaccine (Dr. was senior cardiologist). | |
| COVID19 VACCINE (COVID19) | Fever, Malaise | |
| COVID19 VACCINE (COVID19) | After the 15 min monitoring, I went back to work 15 min later. the left side of my face started tingling which went to a numbing feeling down the left side of my body affecting my neck, shoulder, arm, elbow and up the upper left torso. My face and neck was numb for about 48 hrs and the rest came back to sensation within 24 hrs. D/T the numbness I was admitted into the hospital for a 24hr observation. | |
| COVID19 VACCINE (COVID19) | “””Pfizer-BioNTech COVID-19 Vaccine EUA.”” Patient received first dose of vaccine on 12/21/2020. Patient called on 1/3/2021 to notify that she developed symptoms on 12/27/2020 and had an appendectomy on 12/28/2020 at another facility. Patient also reports that CDC V-Safe Application was used to report event as well. Patient reports that she is recovering well.” | |
| COVID19 VACCINE (COVID19) | Patients adverse reactions started day of vaccination with right arm pain up to right ear as well as complete tongue numbness. On 1-1-21 patient had increased Bell’s Palsy symptoms including; inability to raise left eyebrow, inability to close left eye in its entirety, teeth being numb on left side, and numbness and tingling in left foot and left hand (from palm to fingers). ER physician prescribed on 1-1 Prednisone, Keflex and Valtrex. Patient went to ER again on 1-2-21 with lower extremity numbness on left side that is moving proximally toward her hip. Patient went home on 1-3-21 with an RX for Prednisone as well as Valtrex. Symptoms have improved but have not fully resolved at this time. | |
| COVID19 VACCINE (COVID19) | She was hospitalized on 01/04 but exact situation unknown. COVID +. Hospitalized at Medical Center. | |
| COVID19 VACCINE (COVID19) | Employee received COVID 19 vaccination at 9:45am on 12/30/20. ~15 min. later she developed a rash down her left arm, then down her Rt. arm. about 4 hours later she decided to go to the emergency room for Hearty Palpitations, Fever, Chest discomfort and feeling of generalized sunburn. Later developed severe headache.. | |
| COVID19 VACCINE (COVID19) | Right arm swelling very bad right after shot, next day woke up to get ready to work I started to get light headed, dizzy, sweating, felt like I was going to pass out. My husband then called 911. They took me to Hospital ,I stayed there for a couple hours then released. They told me to stay home and the next day I felt fine. I did a televisit with my Nephrologist (Kidney doctor) the following week. | |
| COVID19 VACCINE (COVID19) | noticed twitching in L arm shortly after receiving vaccine, numbness , weakness and pain in arm and shoulder girdle, diagnosed with parsonage turner syndrome by neurologist, currently taking neurontin for pain as steroids not tolerated | |
| COVID19 VACCINE (COVID19) | Felt slight warmth throughout body about 5 minutes after vaccine. Disappeared 2 minutes later. Arm started to feel sore as the day went on and was very sore by nighttime. Next day, arm started to feel better and over the next 3 days was no longer sore. On the morning of the 30th, woke up feeling fine, took a 3.5 mile walk and felt fine. Around 12:30 pm, experienced sudden pain and a burning sensation in the chest and both upper arms. Thought it was possibly heartburn ; took a Prilosec. The discomfort (mild but steady) continued so checked blood pressure which was 141/91. Called cardiologist and went to emergency room per instruction, around 1:30. Admitted overnight with the diagnosis of a mild heart attack and performed a heart catheterization where they found no major blockage. One artery noted 30% blocked but that overall heart function looked good. Discharged on 12/31/2020. -reported by patient via email, on 1/3/2021 @ 4:49pm | |
| COVID19 VACCINE (COVID19) | Bell?s palsy, right side of face is numb, with difficulty closing eyes, smiling, raising eyebrows, eating, drinking, swallowing. | |
| COVID19 VACCINE (COVID19) | 12/21 had covid vaccine (dose 1). On evening of 12/29 had sudden onset of mild neck pain and significant weakness and numbness of left arm, weak hand grip, clumsiness in hand . Did not improve after trying to shake arm/move around , and took prednisone 40mg oral. Went to ER and had CT Cspine which did not show evidence of cervical pathology. Continued with corticosteroids, sought consultation with PMR and neurology specialists, and steroid dose increased to 60mg/day. Some improvement in strength , but still have diminished sensation and strength in left hand/arm. Unable to perform full job tasks as I am left hand dominant. Likely brachial neuritis / parsonage turner syndrome per both specialists seen. Continuing with corticosteroids at this time, pending bloodwork and OT evaluation | |
| COVID19 VACCINE (COVID19) | Resident exhibited no adverse events during 30 minute monitoring following vaccine administration. Resident found without pulse at 1900. | |
| COVID19 VACCINE (COVID19) | thrombotic stroke -necessitating hospitalization; and craniotomy; required mechanical ventilator for 2 days. Patient now extubated, breathing on her own. Patient remains hospitalized with marked deficits (aphasic) | |
| COVID19 VACCINE (COVID19) | Lightheadedness, throat tightness. Increasing chest tightness. History of atrial fibrillation and bilateral breast implants. Received two doses of epinephrine and one dose of diphenhydramine. | |
| COVID19 VACCINE (COVID19) | Patient developed a septic knee (history of arthroplasty) need for immediate surgery, hospitalization and months to years of antibiotics in his future now. | |
| COVID19 VACCINE (COVID19) | Presented to the ED after developing chest tightness, cough, lightheadedness, and throat closing sensation. She received the Moderna COVID-19 vaccine on the morning of presentation. Within 15 minutes of receiving the vaccine she developed pain and numbness, starting at the injection site traveling down the ulnar aspect of her arm, and nausea. Over the next several hours she continued to develop worsening nausea, chest tightness, cough, lightheadedness, and the sensation that her throat closing. She took PO Benadryl 25mg; however, her symptoms were not alleviated. She was subsequently evaluated in the ED. á Received PO Benadryl 25mg, IV Benadryl 25mg, Epinephrine 0.3mg x 2, IV Famotidine 20mg, IV Solumedrol 125mg & 60mg, DuoNebs x 3, Racepinephrine x 1. | |
| COVID19 VACCINE (COVID19) | Decompensation and temp 103.6. | |
| COVID19 VACCINE (COVID19) | Around 10 or 11 pm, arm pain, chills, fatigue, headache, nausea, swollen lymph nodes, lightheadedness, fainted in tub. Next day, fatigue all day, couldn’t talk, or eat. Went back to bed again. The following day hot flashes, weakness. went to ER. Felt like blood pressure was dropping, tingling in legs, difficulty lifting her head. | |
| COVID19 VACCINE (COVID19) | 20 minutes after receiving the vaccination the resident started to not feel well. She said she felt very far away and just kept repeating I don’t feel well. She was diaphoretic and her chest was very red and she kept scratching and rubbing it at it. I asked if she wanted IM Benadryl or epipen and she at first denied. She also said she felt like she needed to focus on her breathing. At this time we decided it was best to administer Epipen x 1 dose. Immediately after she felt better. She was observed for another 30 minutes and then went home. at 7:17pm I called and spoke with her. She said her arm was sore and that her oxygen levels were about 88-89% which is low for her but she said she felt fine and is currently working right now. | |
| COVID19 VACCINE (COVID19) | Presented to the ED with cc of left sided facial and LUE numbness and weakness x 1 days. Patient received her COVID-19 vaccination on 12/30/2020 around 1PM. Immediately after the injection in her left shoulder, she began to feel warmth and numbness in her left shoulder, arm, neck, face, and chest. She reports later experiencing nausea, palpitations, and left arm weakness. Her symptoms persisted, and her family noted a left sided facial droop which prompted her ED visit. á In the ED, patient was noted to have some left sided facial droop and left arm and leg weakness. CT head and CTA showed no acute abnormalities. Tele-neurology was consulted who recommended admission to rule out acute stroke. Ultimately, work up was negative and symptoms resolved. Symptoms appear to be related to the vaccine. | |
| COVID19 VACCINE (COVID19) | Severe joint aches, fever-type symptoms, nausea | |
| COVID19 VACCINE (COVID19) | Cough began approx 5 min post vaccine, then pt experienced flushing of neck, chest tightness and SOB. Placed on O2 mask at 8L/min, given 1 dose of Epi and transferred to ED | |
| COVID19 VACCINE (COVID19) | On 12/24 at around 10 PM, circulation to my 4th left digit significantly decreased after being outside of my car for around 15 minutes during a temperature of about 50 degrees. I realized when sharp pain was felt at the digit. After about 5 minutes the digit felt numb. I got in my car, turned on the heater, and massaged my finger. Sharp pain was felt again as circulation returned to the digit. The event last approximately 10 minutes from the moment I realized the finger was pale until color returned. This occurred again on 12/27 at around 2 PM as I walked from my car into a store at a temperature of about 40 degrees. This time, discoloration occurred bilaterally on my left 3rd, 4th, and 5th digits and my right 2nd, 3rd, 4th, and 5th digits. The event lasted more than 15 minutes with constant massaging. This has occurred two more times since then, both times occurring bilaterally with minimal exposure to cold. | |
| COVID19 VACCINE (COVID19) | Began experiencing nausea and general stomach pains the morning after receiving the vaccine. After one day of pain and discomfort I woke the following morning (~44hrs after receiving the vaccine) to extreme acute abdominal pain in the lower right abdomen. Went to Urgent Care facility and was diagnosed by CT scan as having acute appendicitis. An emergency appendectomy was scheduled and performed for later that evening. I stayed at the Hospital overnight on 12/24/2020 post operatively on IV antibiotics to recover from the appendectomy. Was discharged from the hospital on 12/25/2020 and have been recovering for about 10 days now with limitted activity. | |
| COVID19 VACCINE (COVID19) | had a positive COVID test; had a positive COVID test; O2 Saturation of 80% / Hypoxia; shortness of breath; He has a CT scan which showed extensive infiltration in the lungs; muscle pain; chills; body aches; low grade fever; cough; This is a spontaneous report from a contactable physician (pulmonary medicine). This physician reported similar events for 2 patients. This is 1st of 2 reports. A 35-year-old male patient received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration on 18Dec2020 at single dose for covid-19 immunization. There were no medical history and concomitant medications. Caller stated that his close friend who was ER physician (front line worker) and within 24 hours after receiving the COVID vaccine, developed COVID or symptoms of COVID. Patient received the COVID vaccine on 18Dec2020 and the same night patient started with a low grade fever, body aches, chills, muscle pain, shortness of breath, cough, O2 saturation of 80% (hypoxia) and was in the intensive care unit now. Patient swore this was related to the vaccine. This patient tested positive for COVID. He had a CT (computerised tomogram) scan which showed extensive infiltration in the lungs in Dec2020. Patient was admitted to the hospital on 24Dec2020 and then was moved to the ICU 2 days later, on 26Dec2020. Caller thought patient had a positive COVID test at another hospital. Caller did know that tested positive at the current hospital on 26Dec2020 which was done to confirm the previous positive test. Caller thought patient had his first positive COVID test either the same day or the next day after receiving the vaccine. Event of O2 Saturation of 80% / hypoxia was reported as hospitalization from 24Dec2020 and life threatening; infiltration in the lungs and shortness of breath caused hospitalization from 24Dec2020, muscle pain, chills and positive COVID test was reported as medically significant; and other events were reported as non-serious. Outcome of O2 saturation of 80% / hypoxia and shortness of breath was not recovered, outcome of cough was recovering; and outcome of other events were unknown. Information about lot/batch number has been requested. ; Sender’s Comments: Based on the information currently available, a lack of efficacy with suspected vaccine BNT162B2 in this patient cannot be completely excluded.,Linked Report(s) : US-PFIZER INC-2020519020 same reporter/drug , different patient/AE. | |
| COVID19 VACCINE (COVID19) | Anaphylactic reaction requiring two doses of Epinephren to control. Still having issues; other vaccine same date product received; This is a spontaneous report from a contactable nurse (patient). A 54-year-old female patient received her first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE), intramuscularly on right arm at 12:30 PM on 18Dec2020 at single dose for COVID-19 immunization, and first dose of other Pfizer vaccine same date product (other vaccine same date lot number: elt9899) on right deltoid on 18Dec2020 for unknown indication. Medical history reported as none. Concomitant medication included vitamin C and colecalciferol (VITAMIN D). The patient experienced anaphylactic reaction at 12:30 PM on 18Dec2020 requiring two doses of epinephren to control. still having issues, resulted in: Doctor or other healthcare professional office/clinic visit, Hospitalization in Dec2020. days hospitalization: 1. The patient received treatment: 2 doses of epinephrine, solumedrol, benadryl, IV and O2 for event anaphylactic reaction to vaccine. The outcome of anaphylactic reaction was not recovered. Lot/Batch and Expiration date has been requested.; Sender’s Comments: The information available in this report is limited and anecdotal and does not allow a medically meaningful assessment of the case. There is a plausible temporal association between vaccines administration and onset of the reported event. It is unclear what is the nature of the vaccine co-administered with BNT162b2. Currently no information is available on the co-administration of the Pfizer-BioNTech COVID-19 Vaccine with other vaccines. | |
| COVID19 VACCINE (COVID19) | Rhonchi, frothy sputum, low grade temp, elevated HR, 12/30 MD assessed 1:00PM and increased prednisone and added Cefdinir. Sent to hospital 12/30 at approximately 6:30PM with worsening symptoms | |
| COVID19 VACCINE (COVID19) | Redness and warmth with edema to right side of neck and under chin. Resident was on Hospice services and expired on 1.1.21 | |
| COVID19 VACCINE (COVID19) | 12/30/2020 07:02 AM Resident noted to have some redness in face and respiration were fast. Resident vital signs were abnormal except blood pressure. Temp at the time was 102.0 F taken temporal. Resident respirations were 22 labored at times. Pulse is 105 and pulse ox 94% on room air. Resident is made comfortable in bed. Notified triage of change in condition also made triage aware of resident receiving Covid vaccination yesterday morning. Resident appetite and fluid consumption has been poor for few days. 12/30/2020 07:32 AM Received order from agency to administer Acetaminophen 650mg suppos rectally due to resident not wanting to swallow anything including fluids, medications and food. This writer administered medication as NP ordered. Will monitor for effectiveness and adverse effects if any. 12/30/2020 08:41 AM Received new orders to obtain Flu swab, obtain CBC and BMP, and Chest Xray all to be obtained today. Notified family of resident having temperature and vital signs excluding b/p that was abnormal. Family was thankful for call and inierated to nurse that family does not want resident sent to hospital. Did educate family on benefits of Hospice services, but family persistant on continued daily care provided by nursing staff. Requests visits if decline continues. Family assured if resident continues to decline, facility will accomandate resident family to be able to be at bedside when time comes to do so. NP ordered IVF and IV Levaquin on 12/31/20. Family chose at that time to sign for Hospice services and not have resident provided with IVF or IV Antibiotics | |
| COVID19 VACCINE (COVID19) | Patient received her Vaccine on 12/16/2020. Afterwards she developed symptoms of fever, chills, diarrhea, nausea, vomiting and headache that became worse over time and on day os presentation to our hospital on 1/2/2021 she was having photophobia. Current headache at time of admission had been persistent for over a week. Patient has no immunocompromising risk factors and was diagnosed with confirmed CMV meningitis. She was also admitted with transaminitis. | |
| COVID19 VACCINE (COVID19) | “The resident received is vaccine around 11:00 am and tolerated it without any difficulty or immediate adverse effects. He was at therapy from 12:36 pm until 1:22 pm when he stated he was too tired and could not do anymore. The therapist took him back to his room at that time and he got into bed himself but stated his legs felt heavy. At 1:50 pm the CNA answered his call light and found he had taken himself to the bathroom. She stated that when he went to get back into the bed it was “”abnormal”” how he was getting into it so she assisted him. At that time he quit breathing and she called a RN into the room immediately. He was found without a pulse, respirations, or blood pressure at 1:54 pm. He was a DNR.” | |
| COVID19 VACCINE (COVID19) | 6-7 hours after the vaccine she developed arm pain, fever and chills. About an hour later she started to have abdominal pain which worsened over the course of the day to excruciating. She went to the Emergency Room where a CT scan revealed a perforation of her sigmoid colon and had a resection of the area of the colon and a diverting colostomy surgery done the evening of 1/3/2021. | |
| COVID19 VACCINE (COVID19) | starting to feel lethargic and weak. Had menses with increased blessed. Called physician to have blood work done to see if I was experiencing anemia. Blood work complete on 12/31/2020. On 1/3/2021, I woke up with blood blisters all over the inside of my mouth and petechia on my trunk and bilateral upper and lower extremities. I called my primary physician to report the symptoms. He suggested to go to the ER if my symptoms worsened. Later that evening I started with a nose bleed and did go to the ER. Upon arrival to the ER, my platelet count was 9. I was admitted to the hospital and diagnosed with ITP. | |
| COVID19 VACCINE (COVID19) | 12/31/20 around 11am Numbness in right hand and right cheek, 5 minutes later, slurred speech. Episode lasted approximately 10 minutes. Treated in ER. Labs, MRI, all normal 1/3/21 Swollen lymph node to left axilla 1/4/21 Rash to injection site | |
| COVID19 VACCINE (COVID19) | Tachycardia, resident was sent out to the hospital for evaluation on 12/30/2020 and came back to the facility on 12/31/2020. | |
| COVID19 VACCINE (COVID19) | Anaphylactic Reaction, facial swelling, facial Redness, Face felt like it was burning, face flushing, throat swelling, heart palpitations, trouble swallowing , feet swelling, light headed, anxiety. Hospitalized from the 12/23/20 to 12/26/2020 . Medications now on Epinephrine, diphenhydramine, cetirizine, famotidine, prednisone, lorazepam, cephalexin. on 1/1/2021 was taken to E.R. by ambulance around 11:00 am left hand was tingle started to go numb traveled up my arm into left side of my face ,ear, tongue, and then down to the left side of my leg and into left foot, could not move left side of body for a good 7 to 8 mins then went away transferred to ambulance enroute to ER blood pressure was high and and started having right ear pain and right side frontal severe headache, arrived to ER and was given diphenhydramine ,ketorolac, metoclopramide HCI, lorazepam. MRI was ordered and Neurologist found two small lesions on right side of frontal brain, following up now with neurologist. added more meds naproxen | |
| COVID19 VACCINE (COVID19) | 2 minutes after vaccine was administered, noticed swelling back of tongue, progressed to posterior 2/3 of tongue, tachycardia, elevated BP. Progressive angioedema involving larynx, cough, shortness of breath. No wheezing. Physical exam did do show any obvious swelling. O2 sat decreased to 80, 1st epinephrine IM administered, 50mg benadryl IV and Famotidine administered. some improvement in symptoms. In 30mins, reoccurrence of angioedema and second epinephrine vaccine administered. Monitored for 2 hours without reoccurrence of symptoms and discharged from ER. | |
| COVID19 VACCINE (COVID19) | palpitations, chest tightness/heaviness, scratchy throat/frequent throat clearing, head heaviness, blurred vision, elevated blood pressure. Evaluated in ED received solumedrol, benadryl. Discharged home and returned to hospital as direct admit due to continued symptoms. Pertinent labs reveled AKI, elevated LFTs. AKI and LFTs improved with IVFs. | |
| COVID19 VACCINE (COVID19) | Found deceased in her home, unknown cause, 6 days after vaccine. | |
| COVID19 VACCINE (COVID19) | Vaccine 12/30/2020 Screening PCR done 12/31/2020 Symptoms 1/1/2021 COVID test result came back positive 1/2/2021 Deceased 1/4/2021 | |
| COVID19 VACCINE (COVID19) | Abdominal pain that proceeded to get worse into the next day. Connected with PCP, had labs drawn and ultrasound ordered. Ended up going to ER. Determined to have Appendicitis, needed and had appendectomy on 12/29/2020. | |
| COVID19 VACCINE (COVID19) | “deceased on 31Dec2020 with no previous side effect; This is a spontaneous report from a contactable physician via “”Pfizer””. An 87-year-old female patient received the first dose of the bnt162b2 (PFIZER-BIONTECH COVID-19 MRNA VACCINE; Lot Number: “”not known because vaccination team vaccinated at care home””), via an unspecified route of administration on 29Dec2020 at a single dose for COVID-19 immunization. The patient’s medical history included upper respiratory tract infection, changing patient weakness; both from an unknown date and unknown if ongoing. Concomitant medications were not reported. The patient experienced: deceased on 31Dec2020 with no previous side effect; which resulted in death on 31Dec2020. The clinical course was reported as follows: the patient received the first dose of the PFIZER-BIONTECH COVID-19 MRNA VACCINE on 29Dec2020; and the patient was deceased on 31Dec2020 with no previous side effect. The patient received the vaccination with a negative COVID-test on 25Dec2020; “”in case of upper respiratory tract infection and changing patient weakness””. The physician reported that “”after good breakfast at 09:13 found without vital signs during routine control.”” The clinical outcome of the event was fatal. The patient died on 31Dec2020 due to unknown cause of death. It was unknown if an autopsy was performed. The batch/lot numbers for the vaccine, PFIZER-BIONTECH COVID-19 MRNA VACCINE, were not provided and will be requested during follow up.; Sender’s Comments: The limited information available does not allow a meaningful assessment by the company. The advance old patient had upper respiratory tract infection, changing patient weakness; further information such as complete medical history, concomitant treatments, particularly death cause and autopsy results are needed for fully medical assessment. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to regulatory authorities, Ethics Committees, and Investigators, as appropriate.; Reported Cause(s) of Death: deceased on 31Dec2020 with no previous side effect” | |
| COVID19 VACCINE (COVID19) | felt like she had a stroke; fell down; Pain in leg; itchiness in her head; left leg not functioning normally; This is a spontaneous report from a contactable nurse (reporting for herself). A 51-year-old female patient received the first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 MRNA VACCINE; Lot number: EH9899), via an unspecified route of administration in the left deltoid on 21Dec2020 at 10:00 (at the age of 51-years-old) as a single dose for COVID-19 immunization. Medical history was none. There were no concomitant medications. There were no prior vaccinations within 4 weeks prior to the first administration of the suspect vaccine. On 21Dec2020, the patient experienced left leg not functioning normally, which was reported with the seriousness criteria of disability. On 21Dec2020, the patient had itchiness in her head. The patient felt like she had a stroke, fell down and pain in leg on 22Dec2020, which were all reported with the seriousness criteria of disability. The patient called the doctor office and spoke with the doctor on call and was told to use diphenhydramine hydrochloride (BENADRYL). No further details provided. The patient was sent home for 10 days and she was sent back to work. The patient underwent lab tests and procedures which included COVID: negative in Dec2020. The outcome of the events was not recovered. The reported assessed the events related to the suspect product, BNT162B2.; Sender’s Comments: The reported events leg dragging, leg pain and fall and suspected stroke were possibly related to the use of first dose of bnt162b2 (PFIZER-BIONTECH COVID-19 VACCINE), due to temporal relationship. However, stroke was not diagnosed. The case will be reassessed should additional information become available. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | PATIENT VACCINATED AROUND 9AM. SHE REPORTS SHE FELT WARM/FLUSHING, FAINT AND STOMACH SPASMS WITHIN ABOUT 4-5 MINS. SHE FELT BETTER AND GOT UP TO WALK ABOUT 30 MINS LATER. SYMPTOMS WORSENED AFTER WALKING ~9:45AM: FAINT AGAIN, SEVERE RETCHING, BP196/140 TO 199/164, TROUBLE SWALLOWING, SOB, WHEEZING. AT 9:58AM, EPI PEN 0.3MG ADMINISTERED AND EMS ACTIVATED. SYMPTOMS REPORTED IMPROVED FOLLOWING EPI. EMS ARRIVED 10:05AM. PATIENT REPORTED RECEIVING 2 BAGS OF PEPCID, STEROIDS, AND ZOFRAN AT HOSPITAL. WAS RELEASED BETWEEN 11:30AM-12PM ON 1/4/21, BP 140/90 AND ACUTE SYMPTOMS RESOLVED. FOLLOW UP WITH PATIENT 1/5/21: NO PRIOR HX OF HTN, BP 120/60, NO SOB/ BREATHING DIFFICULTY. C/O SEVERE HEADACHE, LOW TEMP, FATIGUE, MUSCLE ACHES, SORE THROAT. | |
| COVID19 VACCINE (COVID19) | “Patient had SVT; flushing; hives; heart rate increased to 160’s (had been 180’s earlier in the day); This is a spontaneous report from a contactable pharmacist. A 46-year-old female patient received the first dose of BNT162B2 (lot number: EK5730), via intramuscular, on 28Dec2020 at single dose for COVID-19 immunization. Facility where the most recent COVID-19 vaccine was administered was hospital. The patient was not pregnant at the time of vaccination. The patient was not diagnosed with COVID-19 prior to vaccination. Since the vaccination, it’s unknown if the patient was tested for COVID-19. No other vaccines were received within 4 weeks prior to the COVID vaccine. The patient’s medical history and concomitant medications were not reported. The patient had SVT, flushing, hives 20 min after receiving vaccine on 28Dec2020. Patient was taken to ED and evaluated. SVT resolved. Patient sent home on heart monitor. Later that night while in bed, heart rate increased to 160’s (had been 180’s earlier in the day) and patient was admitted to hospital. Patient is a NP. Treatment received for the adverse event included cold water to face, vagal massage. The outcome of the event””Patient had SVT”” was recovered on 28Dec2020 and of other events was recovering.; Sender’s Comments: A causal association between BNT162B2 and the reported events supraventricular tachycardia, flushing, hives, heart rate increased cannot be excluded based on the compatible temporal relation. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to RAs, Ethics Committees, and Investigators, as appropriate.” | |
| COVID19 VACCINE (COVID19) | a high fever; extreme fatigue; have allergies; This is spontaneous report from a non-contactable consumer. This consumer reported similar events for eight patients. This is the first of eight reports. Only this report is serious. A patient of unspecified age and gender received bnt162b2 (BNT162B2 also reported as Pfizer version of the vaccine, lot/batch number and expiry date were not reported), via an unspecified route of administration on unknown date in Dec2020 at single dose, for Covid-19 immunisaton. The patient medical history and concomitant medications were not reported. The patient got the vaccine at a facility then had a high fever and extreme fatigue after getting the vaccine on Dec2020. The patient was admitted to the ICU. The patient had allergies, but it was unknown what the allergies are to. The outcome of events was unknown. No follow-up attempts are possible. Information on batch/Lot number can not be obtained. No further information is expected.; Sender’s Comments: Linked Report(s) : US-PFIZER INC-2020520700 same reporter/drug/AE, different patients;US-PFIZER INC-2020520703 same reporter/drug/AE, different patients;US-PFIZER INC-2020520699 same reporter/drug/AE, different patients;US-PFIZER INC-2020520704 same reporter/drug/AE, different patients;US-PFIZER INC-2020520705 same reporter/drug/AE, different patients;US-PFIZER INC-2020520701 same reporter/drug/AE, different patients;US-PFIZER INC-2020520702 same reporter/drug/AE, different patients;US-PFIZER INC-2020520700 same reporter, drug, events, and different patients;US-PFIZER INC-2020520701 same reporter, drug, events, and different patients | |
| COVID19 VACCINE (COVID19) | Pt received COVID Vaccine at 1055, 1120 pt began coughing severely and could not stop, unable to speak. 1120 25mg Benadryl liquid given, Pepcid 20 mg given PO, cough worsening. 1122 second dose of Benadryl given, called for MD. Brought pt to Private room via wheelchair. Upon arrival, audible stridor noted. Epinephrine 0.3 mg IM given at 1126. IV started, placed patient on monitor and O2 via 1L NC. MD at bedside along with RT and pharmacy. 1134 Solumedrol 125 mg IV given, 1 puff of Ventolin given. Lungs clear. 1140 Coughing stopped, pt able to speak now. Vital signs: 1130 SPO2 99% Pulse 142 1135 99% pulse 106 BP 168/102 1140 sats 100% HR 93 BP 157/105 1145 sats 100% HR 97 BP 159/93 1200 sats 99% HR 103 155/97 114 | |
| COVID19 VACCINE (COVID19) | Pt vaccinated on 12/23. PCP notified that SOB and fatigue getting worse on 1/4. Unable to keep pre-op Dental work planned prior to mitral valve surgery on 1/14/2021. PCP referred her to our ED where she was diagnosed with COVID-19 and transferred to facility, which is where her surgery was planned. | |
| COVID19 VACCINE (COVID19) | Pt received vaccination and left after 15 min. observation symptom free. He drove a short distance away from the clinical site when he felt profuse sweating and had syncope (seconds) crashing into curb. He was aroused from impact and was able to stop car. He then developed profuse nausea and sudden urge to defecate. He went to restroom. Given these events he returned to the clinical site in another vehicle. Upon arrival he denied chest pain, shortness of breath or ongoing nausea or abdominal pain. He reported his AM blood sugar was 72 and does not take insulin. No history of coronary disease or syncope. EMS was activated and assumed care. | |
| COVID19 VACCINE (COVID19) | The resident who was known to have seizures, and under control for many yeas with Keppra 1000 mg twice a day, on the second day after vaccination developed recurrent seizures requiring hospitalization to an intensive care unit, with intubation and mechanical ventilation until 1/5/21 (to be extubated today). She is still at the hospital. | |
| COVID19 VACCINE (COVID19) | RECEIVED VACCINE ON 12/22; ON 12/24, STARTED FEELING WEAK AND HAVING GI ISSUES WITH DIARRHEA. ON 12/27, STARTED HAVING SHORTNESS OF BREATH AND WHEEZING MORETHAN HER NORMAL WITH HER ASTHMA ILLNESS AND CAME TO ER. WAS TESTED AND FOUND TO BE COVID POSITIVE. | |
| COVID19 VACCINE (COVID19) | Resident received Covid Vaccine, noted after 30 mins with labored breathing BP 161/77, HR 116, R 38, T 101.4, | |
| COVID19 VACCINE (COVID19) | 22 year old patient with no known allergies or medical history admitted 12/21 with TTP and currently being worked up. Currently unclear if related or unrelated to COVID vaccination, but received Pfizer vaccine Thursday 12/17. | |
| COVID19 VACCINE (COVID19) | 51-year-old female with history of intermittent asthma presented to the ED with 1 week of intermittent fevers, myalgias, arthralgias and headache. Patient reports receiving first dose of moderna vaccine last week. She initially developed arm soreness followed by chills and body aches. Subsequently developed frontal headache, photophobia, back pain, nausea and vomiting. She was seen in the ER on 1/1/21, when her work-up including labs, CT spine, chest x-ray were negative therefore she was discharged home. She continued to have symptoms and also developed bilateral intermittent ear pain. She also developed rash in her extremities and torso. Rash is pruritic but not painful. reports ongoing history of neck pain for which she sees PT. Denies sore throat, cough, chest pain or shortness of breath. | |
| COVID19 VACCINE (COVID19) | Vaccine given on 12/29/20 by Pharmacy. On 1/1/21, resident became lethargic and sluggish and developed a rash on forearms. He was a Hospice recipient and doctor and Hospice ordered no treatment, just to continue to monitor. When no improvement of codition reported, doctor and Hospice ordered comfort meds (Morphine, Ativan, Levsin). Resident expired on 1/4/2021 | |
| COVID19 VACCINE (COVID19) | DEATH ON 1/4/2021, RESIDENT RECIEVED VACCINE ON 1/2/20 | |
| COVID19 VACCINE (COVID19) | Began experiencing increased temp of 101.4 on 1/4/2021 at 0701. Temp did not resolve with the use of Tylenol. HR increased to >100 and BP was decreasing below baseline. Increased weakness also noted. Temp increased to 102.9 on 1/4/2021 at 2220. Transferred from SNF to ER for evaluation. | |
| COVID19 VACCINE (COVID19) | Resident had body aches, a low O2 sat and had chills starting on 12/30/20. He had stated that they had slightly improved. On 1/1/21 he sustained a fall with a diagnosis of a displaced hip fracture. On 1/2/21 during the NOC shift his O2 sat dropped again. He later went unresponsive and passed away. | |
| COVID19 VACCINE (COVID19) | Administered first dose of COVID19 vaccine at 1:29pm on 1/4/21. At approximately 11:00pm resident exhibited acute respiratory decompensation with very limited air entry and hypoxemia. Patient received Benadryl, steroids, epinephrine, and Duoneb without improvement. Resident was referred to the emergency room and found to be COVID positive. No fever or rash were reported. | |
| COVID19 VACCINE (COVID19) | LTCF Pfizer Vaccine clinic conducted 12/29/2020 Vaccine lead received a call indicating that a staff member deceased somewhere between 1/3/2021 and 1/4/2021. Cause of death is unknown, and an autopsy is being performed. | |
| COVID19 VACCINE (COVID19) | Vaccine received at about 0900 on 01/04/2021 at her place of work, Medical Center, where she was employed as a housekeeper. About one hour after receiving the vaccine she experienced a hot flash, nausea, and feeling like she was going to pass out after she had bent down. Later at about 1500 hours she appeared tired and lethargic, then a short time later, at about 1600 hours, upon arrival to a friends home she complained of feeling hot and having difficulty breathing. She then collapsed, then when medics arrived, she was still breathing slowly then went into cardiac arrest and was unable to be revived. | |
| COVID19 VACCINE (COVID19) | tremors resident sent hospital facility. | |
| COVID19 VACCINE (COVID19) | Started out vague. Started with headache at the base of her head and then it felt like a web that covered her entire head. By 10:00 she did not feel good. Went to sleep instantly. Slept for about an hour. Began to have nausea. Sunday headache got worse. Started ringing , buzzing sound in her head. Took Zofran because of nausea. Sunday night chest discomfort. Monday had horrible chest pain, headache was horrible, then vomiting. went to ER, doctor felt it was a reaction to the vaccine. was give medicine for nausea and Decadron for the reaction. Gave fluids for dehydration. Got medicine for headache. Keep taking Zofran for nausea. Still has headache. Has appointment with pcp in the morning. | |
| COVID19 VACCINE (COVID19) | “Received COVID-19 Moderna vaccine on 12/28. Developed nausea, vomiting, diarrhea, fever, and hypoxia at facility the day follow vaccine administration (12/29). He was sent to the hospital on 12/29 and admitted for post vaccine fever where he had a chest x-ray that showed infiltrates but WBC count was normal and fever resolved upon admission to hospital. Provider documented “”expected reaction to vaccination in a patient with previous COVID exposure””. He returned to the facility on 12/30.” | |
| COVID19 VACCINE (COVID19) | The resident was found deceased a little less than 12 hours following COVID vaccination, and he had had some changes over the last 2 days. He was 96 and had been on hospice care for a little while. Noone noticed any side effects from vaccine after it was given | |
| COVID19 VACCINE (COVID19) | Within 10 minutes of receiving the vaccine patient began to look sleepy and started to gasp for breath. She called for help and slouched over in a chair and was moved to lay on the floor. Her feet were elevated and 911 was called. Epinephrine was given, patient responded by being able to gasp for breath and her face returned to a more natural state. Within 5 min she began to struggle to breath and epi was given again. Some improvement did occur. EMS arrived and she was taken to the hospital. | |
| COVID19 VACCINE (COVID19) | Bell?s palsy | |
| COVID19 VACCINE (COVID19) | Anaphylactic reaction ( swelling and redness of face and torso, shortness of breath, constriction of airway and dizziness) | |
| COVID19 VACCINE (COVID19) | Pt describes falling with onset of weakness below the hip level about 6 inches above the patella with missing clonus reflex. The pt cannot squat down with associated observable loss of strength, pt is not able to stand up. The pt has fallen 7 times since symptom onset around lunchtime between 1200 and 1300. Pt denies LOC. | |
| COVID19 VACCINE (COVID19) | After three days, couldn’t sleep the whole night. The next day, went to work, came home felt jittery. Close to midnight bp 200/90. Took Clonidine 0.1 and went to ER, bp 180/90. waited for almost two hours bp came down to 141/80. Today, bp is back to normal. Took sleeping medication Zzzquil to go to sleep. | |
| COVID19 VACCINE (COVID19) | Aseptic meningitis, prolonged fever for more than a week, headache, elevated transaminase (ALT is 124). Lumbar tap showed elevated WBC of 23, 76% polys and 24% mononuclear, 25 RBC. Glucose and protein are normal. CSF PCR viral panel is negative. Patient was initially given Acyclovir and was stopped when HSV and VZV PCR were negative. He was given vancomycin IV for Gram positive bacteremia which was later stopped because it was deemed a contaminant. | |
| COVID19 VACCINE (COVID19) | Arm weakness increased each day by post vaccine day 4 arm weak and unable to raise arm, conduct ADLs, painful interrupting sleep. Unable to initiate movement in arm. Use other arm to help move arm. Went to ED on post vaccine day 4. Wbc 12. Crp 4. CT no abscess. Mri on 1/4 shows bursitis. DX SIRVA. Bursa aspirated. Pending cultures. PO MEDROL DOSEPAK. | |
| COVID19 VACCINE (COVID19) | 3 Days after the covid vaccine. I Started to have these symptoms around 1am: fever, chills, body aches, cold sweats. I took ibuprofen fell asleep. Around 330am. Woke up with dizziness, headache, chills. Took tylenol. Fell asleep body was extremely cold esp hands and feet. Woke up around 7am. Still had severe body aches and was extremely dizzy, headache worsening. Took ibuprofen. Woke up around noon. Extremely dizzy with chills and body aches headache still severe. Took tylenol. I was not getting better. went to hospital . It was found I had extremely high wbc and had extremely low blood pressure. Diagnosed as septic shock. | |
| COVID19 VACCINE (COVID19) | Immediate warm rush to my head and body. Heart was beating out of my chest and difficultly breathing. Heart rate spiked to 150 (normal around 55). Hand, legs, and mouth started to go numb. Eventually settled down after about 1 hr. Have not felt normal since which has been 3 days. | |
| COVID19 VACCINE (COVID19) | Patient presented to receive COVID-19 vaccine, received vaccine at approximately 10 am. Patient waited 15 minutes for observation and left observation area without complaining of any sx. Patient returned a few minutes after reporting tongue tingling which eventually got to her lips. . No difficulty breathing or any other sx. No history of allergies. NP/RN administered PO Benadryl 25 mg. As of report of this iReport no additional symptoms or intervention needed. Last vitals: 131/83 75spo2. BP higher than usual per patient, sp02 normal. | |
| COVID19 VACCINE (COVID19) | I was instructed to stay for 30min as i have been anaphylatic to cipro in past. at 30min was told i could leave. while driving home on rt 91 my cheekbones became numb. then slowly a few min later my cheeks became numb. a few min later my lips became numb. as i was driving off exit to rt 5 in longmeadow i developed a lump in my throat. i turned around at top of exit and went back to highway to go to ER. this was approx 1645-1650. i went to ER arrived approx 1655. i was shaking. my bp and pulse were elevated. no tingling or swelling in my face. nurse checked my pupils and my smile and were wnl. no history of bells palsy. i received iv fluids, solucortef 125mg ivp, pepcid 20mg ivp, and benedryl 25mg ivp approx 1840pm. approx 45 min after solucortef numbness better but not gone. it started to come back a little more before discharge, which i let md know. she discharged me with scripts for epi-pen, prednisone, and OTC pepcid and benedryl. follow up with my pcp’s office in am 12/24 at 10am with his NP. total time with facial numbness/lip numbness 29 hours. | |
| COVID19 VACCINE (COVID19) | I woke up with tingling in my right hand and arm, my right side, and down my right leg. I went to the ER at Hospital, and was confirmed to have had a stroke in my left thalmus. | |
| COVID19 VACCINE (COVID19) | Patient woke on 1/3/2021 weak having uncontroled bowels and off and on confusion. | |
| COVID19 VACCINE (COVID19) | Fever, RespDepression & COVID positive REMDESIVIR (EUA) 200 mg x1 then 100 mg daily | |
| COVID19 VACCINE (COVID19) | The patient received the vaccine indicated above. Immediately following vaccination the patient states that they began feeling lightheaded and dizzy. | |
| COVID19 VACCINE (COVID19) | Severe right lower quadrant pain, anorexia over 12 hours. Went to the emergency department. Lab results showed elevated WBC and CT scan showed acute appendicitis. Admitted for urgent surgery: laparoscopic appendectomy. Was hospitalized from 12/26/20-12/28/20. | |
| COVID19 VACCINE (COVID19) | Rapid heart rate, shakiness, headache, rash, scratchy throat, raspy voice, dizziness, extreme weakness |
| COVID19 VACCINE (COVID19) | Sudden death; This is a spontaneous report from a contactable physician and consumer. A 41-year-old female patient received the first dose of BNT162B2 (COMIRNATY; Lot Number: UNKNOWN), via an unspecified route of administration on 30Dec2020 at 0.3 mL single dose for COVID-19 immunisation. Medical history included hypertension. The patient’s concomitant medications were not reported. On 01Jan2021, the patient experienced sudden death. The clinical course was as follows: The patient didn’t experience any adverse event at the moment of inoculation with COVID-19 vaccine or the following days. On 01Jan2021, at lunch time, two days after receiving the vaccine, the patient was found unresponsive in her bed by her partner. The cause of death was unknown. It was reported that an autopsy would be performed in the next days; the results were not yet available. The lot number for the vaccine, BNT162B2, was not provided and will be requested during follow up.; Sender’s Comments: The reported information is limited and does not allow a meaningful assessment of the case. It will be reassessed upon receipt of follow up information. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to regulatory authorities, Ethics Committees, and Investigators, as appropriate.; Reported Cause(s) of Death: Sudden death | |
|---|---|---|
| COVID19 VACCINE (COVID19) | patient was noted to be flushed by residential home staff on 12/30/2020, approximately 2.5hrs after vaccination, fever present at that time. Prior to COVID19 vaccine administration, this patient did have exposure and was close contact with a known case of COVID19 in a residential care employee. Patient was taken to hospital for evaluation of febrile status, had positive COVID19 test at that time, and reported hypotension per residential care staff. | |
| COVID19 VACCINE (COVID19) | PATIENT SPOUSE REPORTS THAT PATIENT RECEIVED VACCINE ON 1/4/2021 AND ON 1/5/2021 PATIENT’S ARM BEGAN TO TURN RED AND SWELL AT THE INJECTION SITE. THE SWELLING AND REDNESS BEGAN TO GO DOWN HIS ARM AND HE BROKE OUT INTO A RASH. PATIENT THEN BECAME SHORT OF BREATH. EMS WAS CALLED AND PATIENT WAS TRANSPORTED TO HOSPITAL, WHERE HE WAS TREATED FOR ANAPHYLACTIC SHOCK TO THE COVID MODERNA VACCINE. | |
| COVID19 VACCINE (COVID19) | Anaphylaxis Narrative: 12/22 received COVID-19 vaccine at 1209 and developed SOB at 12:15. Took her own albuterol inhaler without relief. Transported to ED. PE: red hands with swelling, throat and lip swelling with difficulty swallowing. Later developed headache and dizziness then tachypnea and stridor. Meds given – See section 5 PLUS epinephrine IM and infusion @ 0.05 mcg/kg/min, Alb and ipratropium negs, racemic epi nebs. Admitted to the hospital on 12/22 and still hospitalized at the time of this report on 12/23. She remains on an epinephrine drip and was given methylprednisolone 125 mg IV x 2. No previous history of anaphylaxis. History of Reye’s syndrome as a child when given aspirin. | |
| COVID19 VACCINE (COVID19) | Agitation, Sedation, Anaphylaxis, Rash & HYPOtension | |
| COVID19 VACCINE (COVID19) | Went to the ER on the 31st, my face swelled and chest was covered in hives. Lips swelled. I would get nauseated and I felt like there was acid in my stomach. I also had a headache. I have been taking Zyrtec, Benadryl as needed. | |
| COVID19 VACCINE (COVID19) | The day after the vaccine I had fatigue and body aches. Then on 12-23 fevers of 101.4, chills, body aches, diarrhea, vomiting, productive cough, and profuse sweating. Jan 1st I was in the shower and was dizzy with a headache. Loss of consciousness almost happened and that is when i got out of the shower and laid down. Went to the ED and was diagnosed with bilateral pneumonia | |
| COVID19 VACCINE (COVID19) | I did let the nurse know right after I got the vaccine It did feel like a huge water balloon was sitting on my arm and very heavy at the site. I went to the waiting area and the last 5 minutes and I started feeling weird, kind of getting dizzy and my heart started racing really fast. Am I can tell I was getting really hot and my face was flushed and my heart was racing and my hands started shaking, trembling almost like I couldn’t control it. I’ve never experienced something like this. The part that really scared me was my heart racing like it was going to come out of my chest. They called a code, put me in a wheel chair and took me to the ER (where I work). They took my blood pressure which was really high, they did EKG, blood work and urine test. They kept me there for about five hours, while watching me. And the last weird thing that happened at one point I felt like liquid was running thru my body and my feet were really cold for several hours, It felt like someone was pouring liquid over me. I kept asking the nurse if this is normal and no one knew what to tell me. After everything started slowing back down and it had been a couple hours and the next day i felt like I had a slight head ache and felt out of it, kind of like I had a hangover and of course my arm was really sore. The Doctor in the ER did advise me not to get the second dose as it will be worse than the first one. | |
| COVID19 VACCINE (COVID19) | LEFT SIDED CHEST PAIN, SHORTNESS OF BREATH, FELT WARM AND FLUSHED | |
| COVID19 VACCINE (COVID19) | I got my shot on the 19th and that evening it was like a light switch and I was so tired I went to sleep at 730pm I had severe chills and fever and had to go to bed. The next day I still wasn’t feeling well and I was called in to get covid tested and I went to the ER on the 21st and took a rapid covid test that was positive. I was stable and had good oxygenation and was discharged. I have fever nausea vomiting I also had problems with O2 stat i was in the 80s and realized I was having respiratory failure so I was admitted on the 27th and I’ve been here ever since. I had kinetic storm and infusions my O2 stats were bad and I was sent to the covid unit and put on high flow oxygen and negative for a PE, I’m still on the covid unit but I feel much better today | |
| COVID19 VACCINE (COVID19) | Patient was vaccinated Dec 30, 2020. Prime dose of Moderna vaccine. Observed for full 15 minutes post-injection. No complaints when asked during observation. Released. Subsequently, vaccine clinic staff learned from the patient’s supervisor that on Jan 4, 2021 that the patient had expired on Jan 2, 2021. By report from the supervisor, the patient was found dead at his home. The patient’s primary care provider was unaware of his death when contacted by this reporter today (Jan 6, 2021). Electronic Medical Record without any information since the vaccination. | |
| COVID19 VACCINE (COVID19) | after 20-30 minutes my throat started to get tight, I could not swallow properly, I felt dizzy & my heart was beating fast. | |
| COVID19 VACCINE (COVID19) | anaphylaxis, dyspnea | |
| COVID19 VACCINE (COVID19) | “Client received vaccine at approximately 3:50pm, waited in observational area x30min. Left with husband, stated that she got a few miles down the road and starting experiencing tightness in her chest and flushing. She took 50 mg of Benadryl, 30mg of prednisone and two puffs on her inhaler. She returned to the clinic, upon assessment from nursing she looked extremely flushed and anxious, she stated that she still felt tightness and that she had a history of anaphylaxis once before and had used an epi pen in the past. She had an epi pen with her and questioned whether or not she should give it to herself. BP was 190/68, pulse was normal, respirations normal, she continued to experience tightness and “”not able to catch my breath””, encouraged to use epi pen. She administered epi pen to right thigh at approximately 4:45PM, 911 called. Within a few minutes, she stated she was feeling better, less tightness in the chest, flushing was subsiding. BP at 190/70 at 4:52. EMS on scene at 5:03pm. Vitals normal , EKG normal. Client decided not to transport with EMS.” | |
| COVID19 VACCINE (COVID19) | resident expired 1/1/2021 | |
| COVID19 VACCINE (COVID19) | I started having intermittent chest pain moderate in intensity and palpitations. | |
| COVID19 VACCINE (COVID19) | Resident expired 1/3/21 | |
| COVID19 VACCINE (COVID19) | Migraines, right side of face swollen, nausea, tingling | |
| COVID19 VACCINE (COVID19) | Patient tolerated the vaccine well with no apparent side effects. Ten days later awoke 12:30 AM with severe chest and upper back pain, presented to Med Center where he was found to have an Acute Coronary Syndrome. Transferred to Medical Center where he underwent successful PCI with two drug eluting stents for a 99% mid-LAD stenosis | |
| COVID19 VACCINE (COVID19) | Shortness of breath, fever, fatigue | |
| COVID19 VACCINE (COVID19) | At around 11:40am resident was observed to be unresponsive. resident noted with pulse and respiration. Not in any distress. lung sounds clear. Vital Signs BP162/82 P86 R18 T97.1 O2 Sat 96%, fingerstick is 133mg/dl .Resident received COVID 19 vaccine at 11:25am. O2 via 2l NC initiated. Nurse Stat call, 911 initiated, MD at Bedside. Resident awake and responsive. EMT responded and resident left with EMT to be transferred to hospital, remains awake and not in any respiratory distress. | |
| COVID19 VACCINE (COVID19) | Adult failure to thrive; Chronic hypoxemic respiratory failure; Generalized weakness | |
| COVID19 VACCINE (COVID19) | Patient did not display any obvious signs or symptoms; the vaccination was administered at approximately 10:00 AM and the patient continued throughout her day without any complaints or signs of adverse reaction. Patient was helped to bed by the nursing assistant estimated at around 9:00 PM. The facility received notification from the lab around 11:00 PM that the patient’s COVID-19 specimen collection from Sunday, 1/3/21, detected COVID-19. When the nursing staff went to the room to check on the resident and prepare her to move to a COVID-19 care area the patient was found unresponsive, no movement, no chest rises, noted regurgitated small amount of food to mouth left side, lying on left side. Pupils non reactive. | |
| COVID19 VACCINE (COVID19) | coughing up blood, significant hemoptysis — > cardiac arrest. started day after vaccine but likely related to ongoing progression of lung cancer | |
| COVID19 VACCINE (COVID19) | PATIENT REPORTING ITCHING AT 30 MINUTES POST INJECTION. AT 1.5 HOURS POST INJECTION PATIENT REPORTED ITCHY THROAT AND NUMBESS OF LEFT SIDE OF FACE. AT THAT TIME ADVISED TO GO TO EMERGENCY ROOM. NEXT DAY WHEN I FOLLOWED UP WITH PATIENT, SHE REPORTED HER AIRWAY STARTED TO CLOSE AND SHE RECEIVED EPINEPHRINE, AFTER 5 HOURS HER STARTED TO CLOSE AGAIN AND RECEIVED ANOTHER DOSE OF EPINEPHERINE, WAS RELEASED FROM HOSPITAL ROUGHLY 15-16 HOURS AFTER GOING TO ER. | |
| COVID19 VACCINE (COVID19) | At 10:12 am, Client c/o of sore throat, tightness in throat that relieve quickly, nausea, dry heaves, flushed, light headed and dizziness. Called for EMT. They arrive at 10:25 am and transported her to the local hospital for observation. 01/06/21-Treatment in hospital blood draw, medications given Zofran, Decadron, Benadryl, and Pepcid, and IV fluids. Discharged home at 1244. | |
| COVID19 VACCINE (COVID19) | 5 minutes after injection, my feet and palms itched and I was lightheaded but I tried to shake it off and it faded over the next 10 minutes. I did report it and stayed longer and was ok. Then i went straight home and layed down because i did not sleep well night before (was on call ) i awoke 1 hour post injection dry heaving, very nauseated, mild headache, achy, itchy over different parts of my body and weak. Sat up and my face was getting itchier, lips started to swell, tongue started to swell and itch, throat felt like someone was strangling me, had trouble swallowing and trouble breathing. took 2 benadryls immediately and went out into cold air, thought about calling 911 but got better in 10-15 minutes. never have had a reaction like this in my life. have had hives though in the past. If I would have had an epi pen I would have used it (never have had an epi pen) I was frightened but the benadryl worked and I slept due to the benadryl for 5 hours, when I woke up the benadryl wore off and it started again. took more benadryl, and it improved. before bedtime, the benadryl wore off and I had a hard time swallowing my night time meds like my throat was swollen. Took 2 more benadryls, today I am weak and nauseated and ate very little and feel like my face is still red and itchy. I told my sister and she said she is allergic to PEG which i later noted was in the vaccine. i am very disappointed that I had this reaction- I have desparately wanted this vaccine as a medical worker with a lot of covid patients- I onlu hopr this one shot will protect me enough because it is clear to me that i cannot take this vaccine again. | |
| COVID19 VACCINE (COVID19) | Severe Hypotension, Redness, Warmth and sensitivity all over skin surfaces, lack of responsiveness, low oxygen saturation. | |
| COVID19 VACCINE (COVID19) | At approximately, 1855, I was alerted by caregiver, resident was not responding. Per caregiver, she was doing her rounds and found resident in bed, unresponsive, mouth open, observed gurgling noises and tongue hanging out of mouth. This primary caregiver observed resident at baseline and ambulating after dinner at approximately, 1800 less than an hour prior to incident. This PCG called 911 for EMS and gave report of incident. Resident was taken to Medical Center Emergency Department. At ER, CT scan and X-ray was performed. Per report from ER RN, CT scan and x-ray revealed an intracranial aneurysm and fluid in the lungs. Per RN, resident was still unresponsive and was admitted to Medical Center for observation and comfort measures. This primary caregiver reported to RN, resident recently received the first dose of COVID-19 vaccine on 1/2/21. Primary caregiver received a call from Castle RN at 0700, resident expired at 0615. | |
| COVID19 VACCINE (COVID19) | Three to four hours after vaccine had bruising, major loss of range of motion, severe sharp pain, elevated temp and chills due to reaction of injection site Treatment given 24 hrs later- strong antibiotics, anti inflammatory, exercise, and three days out of work Due to loss of function of left arm due to inflammation | |
| COVID19 VACCINE (COVID19) | Severe shortness of breath, administered inhaler, hydralazine with no improvement. Dr. notified. Sent to ER | |
| COVID19 VACCINE (COVID19) | COUGH, RIGORS, NAUSEA, VOMITING, URINARY URGENCY/FREQUENCY, DYSURIA – FOUND TO HAVE LLL PNEUMONIA, CONCERNING FOR POSSIBLE CYRPTOGENIC ORGANIZING PNEUMONIA | |
| COVID19 VACCINE (COVID19) | PATIENT DEVELOPED PROGRESSIVE NEW DYSPNEA, DIFFERENT FROM HER BASELINE. SHE HAS BEEN HOSPITALIZED TWICE FOR PERSISTENT DYSPNEA AND CENTRALIZED CHEST PAIN, WHICH HAS OTHERWISE HAD NEGATIVE WORK UP. | |
| COVID19 VACCINE (COVID19) | 103.5 Fever that wouldn?t come down with Tylenol, chills, sharp headache, tachycardia, site pain, dizziness, body aches, nausea All symptoms started 11 hours after first dose of vaccine (3AM), went to hospital 15 hours after symptoms started and was treated for 9 hours until all symptoms abruptly stopped | |
| COVID19 VACCINE (COVID19) | anaphylaxis; This is a spontaneous report from a contactable other healthcare professional (patient). A patient of unspecified age and gender received bnt162b2 (PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration on an unspecified date at single dose for immunization. The patient’s medical history and concomitant medications were not reported. Pfizer covid vaccine caused anaphylaxis on 30Dec2020 and landed patient in hospital. Patient was an anesthesiologist and had high IgG to thyroid with hashimotos. Patient thought he/she had IgG/neutrophil mediated anaphylaxis (not the typical IgE) as absolute neutrophil count elevated but everything else normal in labs. Had tachycardia into 140-150s and mild facial and throat edema. Still having sudden bouts of elevated heart rate in the morning of 31Dec2020. Heart tests all normal. Outcome of event elevated heart rate was not recovered, and outcome of other events was unknown. Information on the Lot/Batch number has been requested.; Sender’s Comments: A possible contributory role of the suspect products cannot be excluded for the reported events based on the known safety profile and temporal association. Case will be reevaluated based on follow-up information | |
| COVID19 VACCINE (COVID19) | 12/29/2020 2 hr after vaccination patient became hypotensive, decreased oxygen levels was transferred to Hospital currently inpatient at hospital – admitted for cardiac arrest | |
| COVID19 VACCINE (COVID19) | Deceased | |
| COVID19 VACCINE (COVID19) | Woke up Thursday am with hives on right lower abdomen and leg getting progressively worse throughout the day. By that afternoon had back pain in right back and continuing hives. Woke up Friday with numbness to right leg, hives, and back pain all on right side of body. Had numbness to foot, face but especially thigh, back and across upper buttocks. Saturday hives subsiding, numbness receding to face, upper thigh and foot only on right side of body. Sunday, back pain some improved, no hives or hives minimal, numbness persists upper thigh face and foot on right side of body. Monday, Tuesday and Wednesday the same. Woke up Thursday with shingles rash to upper thigh back, numbness to foot face and upper thigh persist only on right side of body. Darn!!! | |
| COVID19 VACCINE (COVID19) | I woke the next morning with flu like symptoms my arm was hurting, I was feelin tired and really couldn’t get out of bed. As the day progressed I got chills, started running a fever, It went up to 104 and my heart rate went up and down from 120-165 so I went to the ER. They gave me fluids and ran a whole bunch of test, tested me for Covid and Sepsis and everything came back normal and negative for Covid. They monitored my heart rate and once it started getting back to normal, they ended up letting me go home, I was there from 8pm to about 3am. After that I felt tired and felt like when I had Covid back in November. I started feeling better about Sunday, still a little tired but felt more back to normal. My heart rate is still getting back to normal so my Dr is following me on that. | |
| COVID19 VACCINE (COVID19) | PT was found deceased in his home on 1/5/2021 | |
| COVID19 VACCINE (COVID19) | Expired 1/05/2021 | |
| COVID19 VACCINE (COVID19) | cardiac arrest; This is a spontaneous report from a contactable physician. A 64-year-old male patient received BNT162B2 (PFIZER-BIONTECH COVID-19 mRNA VACCINE), via an unspecified route of administration on 30Dec2020 as single dose for covid-19 immunization. Medical history included asthma and a little overweight from an unknown date. The patient’s concomitant medications were not reported. The patient experienced cardiac arrest on an unspecified date, which was serious as it lead to death. The patient died on an unspecified date. It was not reported if an autopsy was performed. This batch/lot number is not available despite the follow-up attempts made. No further information is expected.; Sender’s Comments: The reported information is limited and does not allow a meaningful assessment of the case. It will be reassessed upon receipt of follow up information. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to regulatory authorities, Ethics Committees, and Investigators, as appropriate.; Reported Cause(s) of Death: cardiac arrest | |
| COVID19 VACCINE (COVID19) | Moderate to severe headache 24-48 hours post injection. Complete sensorineural hearing loss in left ear 1 week after injection; Moderate to severe headache 24-48 hours post injection. Complete sensorineural hearing loss in left ear 1 week after injection; This is a spontaneous report from a contactable physician (patient). A 37-year-old female non-pregnant patient received her first dose of bnt162b2 (PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration on 22Dec2020 17:15 at single dose on her left arm for covid-19 immunization. Medical history included known allergies to penicillin. The patient had no other medical history. There were no concomitant medications. The patient did not receive any other vaccines within 4 weeks prior to the COVID vaccine and the patient was not received list of any other medications within 2 weeks of vaccination. Prior to vaccination, the patient was not diagnosed with COVID-19. Since the vaccination, the patient had not been tested for COVID-19. The patient experienced moderate to severe headache 24-48 hours post injection. Complete sensorineural hearing loss in left ear 1 week after injection on 23Dec2020. These events resulted in doctor or other healthcare professional office/clinic visit, disability or permanent damage. The patient had received prednisone to treat the events. The outcome of the events was not recovered.; Sender’s Comments: A possible contribution role of first dose of bnt162b2 (PFIZER-BIONTECH COVID-19 VACCINE) to the onset of sensorineural hearing loss in left ear and headache cannot be excluded due to temporal relationship. The case will be reassessed should additional information become available. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | numbness and weakness in left arm; numbness and weakness in left arm; had a brachial plexus pathology; her grip and fine motor are affected in her left arm/she could not do her job; This is a spontaneous report from a contactable physician (patient). A 35-year-old female patient received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE, lot# EH9899), via an unspecified route of administration in right arm on 21Dec2020 at single dose for Covid-19 immunisation. Medical history included ongoing birth control. No other medical history. Concomitant drug included other medication she took for birth control. On 29Dec2020, the patient experienced numbness and weakness in left arm, had a brachial plexus pathology, went to the emergency department on 30Dec2020 and was seen by one of the facility doctors and stated this doctor had her on steroids for treatment. She got the vaccine in her right arm, stated her grip and fine motor are affected in her left arm. States this was disabling since she could not do her job. She was following up with neurology on Monday (unspecified), that she had a CT scan of her neck and it was normal. Only other medication she was taking was for birth control, but she did not feel like it was relevant. The outcome of events numbness and weakness in left arm was recovering, while outcome of other events was unknown. This case was reported as serious, seriousness criteria was disabling.; Sender’s Comments: Based on the information currently provided, the vaccination with BNT162B2 might play a contributory role in triggering the onset of the reported events. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | Right sided facial & top lip Numbness & recurring pain; Right sided facial & top lip Numbness & recurring pain; Right sided facial & top lip Numbness & recurring pain; This is a spontaneous report from a contactable consumer reporting for herself. A 66-year-old female patient (not pregnant at the time of vaccination) received first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE, Lot number EK9231), via unspecified route of administration on 29Dec2020 13:30 on left arm at single dose for COVID-19 immunization. Facility where the most recent COVID-19 vaccine was administered in Hospital. Medical history included Hypertension, Reflux and low back pain. Concomitant medications included ibuprofen, dicycloverine hydrochloride (BENTYL), Valsartan and omeprazole. The patient previously took codeine, Bactrim, Zyrtec and experienced allergies. The patient experienced Right sided facial and top lip Numbness, recurring pain on 31Dec2020 16:00. All events resulted in emergency room visit and Hospitalization on 31Dec2020 for 1 day. The patient underwent lab tests and procedures, which included Cat scan, MRI, ultrasound of heart and labs on unspecified dates, Nasal Swab/Covid 19 test on 01Jan2021 with negative result. The outcome of the events were not resolved. | |
| COVID19 VACCINE (COVID19) | Felt sharp pain under jaw, then facial numbness. Then quickly developed facial swelling, left eye swelling and tongue swelling. Then felt like throat closing up within 7 minutes of receiving vaccine. Received vaccine in the clinic, was transported to Emergency Room. In ER received Epinephrine, steroids, Benadryl and Pepcid. | |
| COVID19 VACCINE (COVID19) | 1 day after the vaccine he had a low grade fever… 1 week later he had a seizure and then multiple ones in the ER… | |
| COVID19 VACCINE (COVID19) | Developed shortness of breath, swelling of tongue, persistent cough within 5 minutes of vaccination. Was treated with EpiPen and kept in ER for observation overnight. Symptoms resolved. | |
| COVID19 VACCINE (COVID19) | Patient contacted provider 12/26 with following symptoms: Dry cough, diarrhea, fatigue for 7 days. Covid test ordered and positive. presented to ED on 12/31 and admitted into hospital. Still inpatient as of 1/7/2021. | |
| COVID19 VACCINE (COVID19) | Patient experienced an episode of SVT and then sinus tachycardia for approximately 6 hours after injection | |
| COVID19 VACCINE (COVID19) | “Pt last seen at 1200 by nurse for ID band check. No visible signs of distress noted. Pt states “”I just want to be left alone””. 1230 nurse was called to pt room. Pt was noted unresponsive, no pulse and respiration noted. CPR started immediately, at 1239 first shock given. 1245 EMT took over, at 1319 EMT called time of death” | |
| COVID19 VACCINE (COVID19) | Hemmoragic Stroke. Began with vision difficulty in the morning. Then I noticed she had left sided neglect. Went to ER. Treated with Andresxa (to counteract Elaquis). In SICU for 2 nights then telemetry unit for 3 nights. CUrrently in Rehab. | |
| COVID19 VACCINE (COVID19) | “Following vaccination the patient had progressively worsening abdominal pain over the next 24 hours. Presented to the ER and was initially thought to have appendicitis. However, it was then discovered during surgery that the appendix was surgically absent. The surgeon did not that the patient did have a “”Round, infarcted ligamentous tissue was wrapped around ascending colon. “”” | |
| COVID19 VACCINE (COVID19) | Patient developed hypoxia on 1/4/2021 and did not respond to maximal treatment and passed way on 1/5/2021 | |
| COVID19 VACCINE (COVID19) | When vaccine was administered, seemed high on my arm. I had immediate soreness and shoulder discomfort, I was told this was normal. It continued to progress and I eventually had decreased ROM, weakness and sharp shooting pain in my shoulder. Working at OI, I consulted provider, xrays were obtained and I was evaluated. He strongly suggested an MRI be obtained as well. That was completed the same day as my evaluation on 12/31/2020 (1 week and 2 days after the vaccine was administered). The provider informed me that they have had patients with similar situations that were evaluated for frozen shoulder after having a vaccine d/t administration site and vaccine going into subacromial space. He does report that this was my case/situation, upon my exam, I had severe inflammation with this as well-he is now having me follow up for a surgical consultation for my shoulder to be repaired. Today’s date is 1/7/2021, I have these same ongoing symptoms that have continued since day of administration, without diminishing in severity. He is unable to provide an injection d/t my upcoming second dose of the COVID vaccine this next week, 1/12/2021. He strongly suggests that my 2nd vaccine be administered elsewhere-advised NOT be administered in the same shoulder OR in opposite to cause these symptoms to flare. He advised in gluteus if possible to avoid any further issues if at all possible. | |
| COVID19 VACCINE (COVID19) | patient declined 12/30/2020 and was transferred to hospital where he did not respond to treatment and passed away 1/4/2020 | |
| COVID19 VACCINE (COVID19) | Patient did not report any signs or symptoms of adverse reaction to vaccine. Patient suffered from several comorbidities (diabetes and renal insufficiency). Patient reported not feeling well 01/06/2021 and passed away that day. | |
| COVID19 VACCINE (COVID19) | Guillain Barre syndrome/AIDP event. Paresthesia and nerve pain developed in bilateral legs 4 hours after shot and progressed slowly for 4 days in intensity and area involved. Symptoms progressed distally to superior. On the 5th day symptoms progressed rapidly and involved bilateral legs up to the groin, left arm up to lateral shoulder, and right hand. I went to the hospital and was admitted to start IVIG treatment for Guillain Barre Syndrome/AIDP. | |
| COVID19 VACCINE (COVID19) | Vaccine Candidate received vaccine approxat 2:30pm, was monitored for 15 min no complications at the time, went home. Around 5:30pm while walking into her home she became unresponsive, was assisted in a siting position, became incoherent, mumbling and started to convulse to the right side of her r upper extremity. Foaming at the mouth and stopped breathing, CPR was initiated for 1-2 min, EMS arrived was transported to Medical Center. She was admitted and is currently hospitalized. MD reports this event is highly unlikely related to the vaccine given her medical history but suggested to report being its a new vaccine. Current status: stable. | |
| COVID19 VACCINE (COVID19) | Person had a fever of 102.4, pulse rate of 118, he was non-responsive with edema of the right calf & ankle this morning when he was assessed. | |
| COVID19 VACCINE (COVID19) | Resident had the COVID vaccine 12/30/2020. 12/31/20, resident has been in bed all shift. Staff became concerned when resident was not easily aroused. Resident displayed signs of tremors, twitching, confusion, in and out of consciousness, low O2 sats, elevated pulse and fever, fatigue and weakness. Writer called NP. NP stated this is most likely a reaction d/t the COVID vaccine. She gave orders for Benadryl 25mg IM x1 now and Tylenol 1000 mg now. NP also stated resident will not be getting the second dose of vaccine. Will continue to monitor and update NP if worsening symptoms. After receiving Benadryl and Tylenol at 145pm, resident began to appear as though she was feeling better and was talking to talk, fever had gone down. Tonight resident is not easily aroused, lethargic, continues to have tremors and twitches, almost appearing as convulsions. When asked if she knows where she is or what day it is, resident can properly answer. Resident denies SOB but staff has noted loud squeals while breathing. NP was updated and gave new orders to give Benadryl 25 mg IM x1 if needed and Ok to send resident to ED. Resident currently refuses to go to the hospital. Will continue to monitor. BP 152/112, P 116, T 99.1, O2 87-91. Resident’s O2 at 1205am was 80% on 3LPM. Resident unable to be aroused from sleep by writer. NAR called to assist. NAR could not arouse resident. Writer and NAR attempted to reposition resident and resident’s breathing became more labored. Resident turned back to previous position and writer called on call MD at approx. 1220am. MD returned call approx. 1235am with orders to send resident to ED. 911 called and ambulance arrived about 1245am. History of present condition given to EMTs and they stated resident would be going to Hospital. Writer has attempted to contact Hospital ED x3 but have been unable to get through. An EMT did just call to clarify when vaccine was given, what symptoms have been present and when they started. She said she has everything she should need and she will let Hospital ED staff know to call if they need anything else. Writer will again attempt to contact them though. Resident’s temp was 97.5 and BG 128. When EMTs arrived they got an O2 reading of 60%. Resident did open her eyes a couple times during transfer from bed to stretcher and while stretcher was going outside but no responses from resident were made. | |
| COVID19 VACCINE (COVID19) | had a vaccination on 12/31/2020 late morning passed away early morning 01/01/2020. This is a 93 year old with significant heart issues. EF of 20% among other comorbidities. He died suddenly approximately 0430, it is unlikely it was related to receiving the vaccine. | |
| COVID19 VACCINE (COVID19) | Immediate pain and loss of range of movement of left shoulder. Physical examination today demonstrates a healing injection site which is fairly superior on the left shoulder, and abduction of the left shoulder which is limited secondary to pain. Patient’s physician’s impression is that he has a subdeltoid bursitis which was temporally associated to the COVID-19 vaccination. (SIRVA) | |
| COVID19 VACCINE (COVID19) | Diffuse polyarthropathy starting the day after vaccination and continuing for 7+ days. Currently treating with abx for concern of possible cellulitis, and prednisone 60mg for polyarthropathy. Currently admitted. | |
| COVID19 VACCINE (COVID19) | woke up with fever and sore throat on 22nd; went to job and got tested and tested positive; on the 23rd developed right upper lobe pneumonia; on 27th was hospitalized with three lobe pneumonia; On 22nd received got zpack – azithromycin and sudafed; received at hospital doxicycleln IV ; ivermectin and went home with them, as well. Hospital | |
| COVID19 VACCINE (COVID19) | Day 2 (12/29/20): Fever (<100 degrees), Mild muscle aches, Fatigue Day 3 (12/30/20): Fatigue, Muscle aches Day 4 (12/31/20): Alternating chills and profuse sweating starting at 8am, Full body flushing, Grand Mal Seizure at 4:30pm | |
| COVID19 VACCINE (COVID19) | Patient was vaccinated at 11am and was found at the facility in his room deceased at approximately 3:00pm. Nurse did not have cause of death | |
| COVID19 VACCINE (COVID19) | Nausea, hives, anaphylactic shock, throat swelling, hypotension, headache, dizziness, weakness . The symptoms returned at 1:25pm the best day as well. I?ve now had two anaphylactic reactions | |
| COVID19 VACCINE (COVID19) | No adverse effects noted after vaccination. Patient with cardiac history was found unresponsive at 16:45 on 1/6/21. Abnormal breathing patterns, eyes partially closed SPO2 was 41%, pulseless with no cardiac sounds upon auscultation. CPR and pulse was regained and patient was breathing. Patient sent to Hospital ER were she remained in an unstable condition had multiple cardiac arrest and severe bradycardia and in the end the hospital was unable to bring her back. | |
| COVID19 VACCINE (COVID19) | 4 days later after the vaccine my left eye turned really red with crust and mucus coming out. I went to walk in clinic and was being treated for pink eye. I was started on an antibiotic eye drop. A day later my right eye started to have the same problem. I scheduled an appt with a eye specialist where he examined both eyes and said I had a major infection. I was started on steroid eye drops which I am still taking but seems my eyes are not getting better. I have a follow up with another specialist next week for further testing. I have been out of work due to this matter. | |
| COVID19 VACCINE (COVID19) | Anaphylactic reaction; hives in the first 10 minutes of the vaccine; This is a spontaneous report from a contactable Other Health Professional (Physician Assistant). A 29-year-old female patient received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration on 29Dec2020 at a single dose for COVID-19 immunization. The patient’s medical history and concomitant medications were not reported. The reporter stated that she is treating a patient in ICU that got the COVID-19 vaccine on 29Dec2020. The patient developed hives in the first 10 minutes of the vaccine and had an anaphylactic reaction 1 hour later. Seriousness of events was reported to be hospitalization. Outcome of the events was unknown. The reporter also mentioned that it was not a mild reaction and patient was still in the ICU, 48 hours later. Information about lot/batch number has been requested.; Sender’s Comments: A possible causal association between administration of BNT162B2 and the onset of anaphylactic reaction and hives cannot be excluded, considering the plausible temporal relationship and the known adverse event profile of the suspect product. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | tested positive for Covid test; tested positive for Covid test; difficulty breathing; chills; fluctuating fever; nausea; weakness; weakness/extreme fatigue; loss of taste and smell; loss of taste and smell; muscle pain; cough; sore throat; nasal drip; dizziness; fast heartbeat; injection site pain; anxiety; crying; This is a spontaneous report from a contactable healthcare professional. This 21-year-old female patient reported for herself that she received BNT162B2 1st dose on 31Dec2020 10:00 AM intramuscular at left arm for COVID-19 immunisation. Medical history included known allergies: Penicillin and Covid-19. Concomitant therapy included BC as reported. The patient experienced difficulty breathing, chills, fluctuating fever, nausea, dizziness, weakness, fast heartbeat, tiredness, loss of taste and smell, muscle pain, injection site pain, anxiety, cough, sore throat, nasal drip, crying, extreme fatigue, Etc on 31Dec2020 at 06:00 PM. The events resulted in doctor or other healthcare professional office/clinic visit, emergency. The patient was hospitalized for 1 day and received treatment included blood thinner rivaroxaban (XARELTO) and had 2 weeks quarantine. The patient had Covid prior to vaccination and tested positive for Covid test post vaccination on 01Jan2021. The outcome of the events was not resolved. Information on Lot/batch number has been requested.; Sender’s Comments: Based on available information, a possible contributory role of the subject product, BNT162B2 vaccine, cannot be excluded for the suspected LOE, SARS-CoV-2 test positive and the other reported events due to temporal relationship. Of note, it is reported that the patient had history of COVID 19 infection prior to the vaccination. There is limited information provided in this report. Additional information is needed to better assess the case, including complete medical history, diagnostics, counteractive treatment measures and concomitant medications. This case will be reassessed once additional information is available. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | Abdominal pain, chills, n/v, dark urine, elevated LFT’s, Bilirubin in urine. Patient currently admitted to hospital | |
| COVID19 VACCINE (COVID19) | Received Moderna vaccine on 12/29/2020. 12/30/2020 fever of 100.4 Tylenol given and monitored and fever went down. 12/31/2020 Chest x-ray completed and sent to the hospital and admitted with pneumonia. 1/3/2021 reported by the hospital that Covid-19 results were positive. He had had Covid-19 positive results back on 11/4/2020 prior to vaccine. | |
| COVID19 VACCINE (COVID19) | “Pt experiencing and c/o left nasal burning, left upper lip tingling progressing to numbness with slight swelling noted, scattered patchy hives to upper front chest, sharp HA above right eye, denies SOB, no acute respiratory distress noted or reported, slurring of words shortly after onset of other symptoms. Pt repeating “”something ain’t right””. Pt received Moderna COVID vaccine at 4:35pm with no reactions or side effect noted within the post 15 and 30 minutes. EMS notified at 5:49pm. MD notifed and ordered Benadryl 50mg IM (given at 5:53pm), EpiPen and DepoMedrol 40mg IM if needed. No respiratory distess noted, pt denies SOB. EMS arrived and transported pt to ER.” | |
| COVID19 VACCINE (COVID19) | Developed SOB and fatigue 1 day after vaccine, went to urgent care and tested positive for COVID at urgent care. Returned to our ED after going to urgent care again on 1/7/21, had o2 sat of 84% on room air, impoved to 98 on 3 liters. Was transferred to another facility for admission. | |
| COVID19 VACCINE (COVID19) | vomiting later on 01/05/21. Lethargy and hypoxia in pm of 01/06/21. Hypotension am of 01/07/21. Hospitalized, intubated, cardiac arrest, died 01/07/21. | |
| COVID19 VACCINE (COVID19) | Swollen lips/tongue, shortness of breath, cough, hives, nausea, headache Epi shot, Benadryl, Pepcid, prednisone | |
| COVID19 VACCINE (COVID19) | Less than 5 minutes after vaccine, nose drained, weird taste in mouth, tingle in nose and on tongue. Throat and tongue swelled, couldn?t speak. Dizzy and slurring speech. Was taken to ambulance outside, BP was 191/101. Given beta blockade. Confused and dizzy for next 2 hours in ER. Evaluated for stroke and given a 12-lead ECG. Given benedryl and prednisone. Felt better after 3 1/2 hours. Continued steroids for 5 days and had to take benedryl every 4 hours for 3 days or swelling/itching/bad taste in mouth would return. Sore arm on day 3. | |
| COVID19 VACCINE (COVID19) | symptoms:chest and stomach pain Has markedly elevated liver function tests that were normal 2 weeks prior to immunization Is being admitted the hospital to monitor liver function test. | |
| COVID19 VACCINE (COVID19) | headache, sore throat, runny nose, arm pain that migrated to the axilla and down the side of the body, joint pain ( hands, wrist, feet, hips, knees, spine, neck), insomnia, general malaise, fatigue, and lower grade fever. Most symptoms lasted about 7 -10 days. However, it is now day 20 after the initial vaccine and I still have joint pain that has not gone away. esp in hands, wrists, and feet. When I sleep I still wake up with all my joints hurting it gets better as I start moving but the wrist, hands, and feet pain has not gone away. This pain will wake me in the night when I change positions. I called my doctor today to inquire if it is a good idea if I should take the second dose because the first dose made me so dibiliated. Awaiting for a response. I am due to take the second vaccine on 2/9/21. | |
| COVID19 VACCINE (COVID19) | Fever, coughing, drowsiness, generalized weakness. Was found to be hypercalcemic (corrected calcium 14) and admitted 1/2-4. No prior history of hypercalcemia. | |
| COVID19 VACCINE (COVID19) | Congestion Shortness of breath Tachycardia Transferred out 911. Per hospital, patient had a myocardial infarction, is unresponsive, and on hospice services. | |
| COVID19 VACCINE (COVID19) | Anaphylactic reaction, Severe edema and raised red rash entire body, Severe itching ,Soft tissue edema of throat. Swelling of, eyes, lips, face. Multiple trips to ER, treated with steroids, Benadryl, prevacid. , CURRENTLY IN ICU ON EPINEPHRINE DRIP, STEROIDS, MULTIPLE MEDS | |
| COVID19 VACCINE (COVID19) | Resident passed away in her sleep | |
| COVID19 VACCINE (COVID19) | Patient c/o fatigue and cough on 1.4.2021 and was encouraged to be tested for COVID. We were notified that the patient was hospitalized on 1.7.2021 with COVID symptoms and positive test results. She is currently on a ventilator and dialysis. | |
| COVID19 VACCINE (COVID19) | On December 25th I had mild chest pain and then on January 1st, 2021 I had severe chest pain that persisted and on January 3rd I was admitted into the hospital. My Ddimer was elevated and my Troponin levels were elevated. An angiogram was performed and Dr. injected nitro into my arteries because they were constricted from Coronary Spasms. | |
| COVID19 VACCINE (COVID19) | Sxs started 3-5 minutes post vax. Dizziness, hypotension, throat fullness, CODE called, given IM Epi at vax site. Taken to ED from vax site. Started on epi drip. Admitted to SHC. | |
| COVID19 VACCINE (COVID19) | Nausea/ dizzy, Syncope 12 hours later. | |
| COVID19 VACCINE (COVID19) | Patient felt warm with palpitations 5 minutes after vaccine administered. was monitored for 30 mins & then returned to work. on 12/30/2020 patient was at work in Presurgical testing dept & experienced near syncope, dizziness & elevated BP. reported to ED & was admitted to telemetry unit. | |
| COVID19 VACCINE (COVID19) | Dr. called this morning and reported that an employee that works in billing had her vaccine on Wednesday and developed and anaphylactic reaction to Moderna. This was 24 hours later with rash, SVT heart rate above 140, low grade fever, redness at site. Admitted and treated with steroids and Benadryl. | |
| COVID19 VACCINE (COVID19) | Came to ER on 12/20/20 with chills, heart palpitations, body aches and increased SOB. Had ST elevation on EKG in ER, taken to Cath Lab- no intervention done. D/C home 12/22/20. Previous Hx of COVID per patient | |
| COVID19 VACCINE (COVID19) | Initial event was soreness at site which resolved on its own within a few days. 2 days after receiving vaccine, I began having an allergy reaction to the same brand N95 that I had been utilizing since the beginning of the pandemic. Symptoms are swollen cheeks and welts , sudden itchiness at the site of my mask placement. The reason for this report is a sudden onset of excruciating and debilitating pain throughout my body specifically pain of my right shoulder radiating down my sprightly arm. I have been receiving testing and treatment for ongoing neuropathy due to Longhauler syndrome, however This recent pain is so debilitating, I spend most of my time in bed. I have been experiencing chills then profuse sweating. I also so fatigued, I sleep much of the day. I have been having episodes of tachycardia with chest tightness which has increased since after having the vaccine. I also become short winded on exertion. I?ve been waking up in a panic and sweating. | |
| COVID19 VACCINE (COVID19) | Patient received first dose of Pfizer COVID-19 vaccine on December 26. On the next day, December 27, patient started having pressure in her head and sinuses, weakness. Then she developed nonproductive cough and progressive shortness of breath. She was seen at urgent care and tested positive for COVID-19 on December 29. She had low oxygen saturation on home oximeter and severe shortness of breath. Patient’s husband is also ill with COVID-19 at home. Patient was sent to the ED and admitted to the hospital. | |
| COVID19 VACCINE (COVID19) | Patient had been diagnosed with COVID-19 on Dec. 11th, 2020. Symptoms were thought to have started on 12/5/2020. Received Moderna vaccine on 12/23. Unexpected death on 1/8/2021. Resuscitation attempts unsuccessful | |
| COVID19 VACCINE (COVID19) | Atrial fibrillation; This is a spontaneous report from a contactable physician downloaded from the Regulatory Authority. The regulatory authority report number is GB-MHRA-EYC 00236011. An 87-year-old female patient received BNT162B2 (PFIZER-BIONTECH COVID-19 mRNA VACCINE; Lot number EJ0553), intramuscular on 18Dec2020 at 0.3 mL, single for covid-19 immunization. Medical history included ongoing hypothyroidism, ongoing diabetes, ongoing atrial fibrillation, ongoing frailty and, ongoing osteoporosis, all from unknown dates. Concomitant medication included prednisolone (MANUFACTURER UNKNOWN), levothyroxine (MANUFACTURER UNKNOWN), salbutamol (MANUFACTURER UNKNOWN), omeprazole (MANUFACTURER UNKNOWN), doxycycline (MANUFACTURER UNKNOWN). The patient experienced atrial fibrillation on an unspecified date, which was serious as it was medically significant, involved hospitalization and lead to death. Clinical course was as follows: the patient was vaccinated. Consent was obtained and a pre immunization checklist was completed. She was observed following the administration of the vaccine, and no adverse effects were noted. She returned home. She became unwell and was admitted to hospital approximately 24 hours later. The patient was admitted to the hospital 24 hours following the vaccination, and subsequently died later, while in the hospital. The full clinical details were unknown, but the diagnosis from Accident & Emergency was atrial fibrillation. It is not clear if this had any relation to the vaccine that was administered, but could not be excluded, per the reporter. The patient died on 20Dec2020. It was not reported if an autopsy was performed. No follow-up activities are possible. No further information is expected.; Reported Cause(s) of Death: Atrial fibrillation | |
| COVID19 VACCINE (COVID19) | Death; Loose stools; Vomited; This is a spontaneous report from a contactable other healthcare professional by Pfizer from the Regulatory Agency (UK-MHRA). The regulatory authority report number is GB-MHRA-WEBCOVID-20201230164020. An elderly female patient received BNT162B2 (COVID-19 MRNA VACCINE BIONTECH, Batch: EJ1677, Expiration date: Feb2021) via an unspecified route on 29Dec2020 at single dose for Covid-19 vaccination. Medical history included dementia and a history of urinary tract infection and delirium, all from an unknown date and unknown of ongoing. Concomitant medication included influenza vaccine (INFLUENZA VIRUS, Batch: 4924B1A) for influenza immunization. Patient has not had symptoms associated with COVID-19. Patient is not enrolled in clinical trial. No known allergies. The patient had not tested positive for COVID-19 since having the vaccine. On the 29Dec2020 the patient experienced loose stools and vomited. The patient underwent lab tests and procedures which included COVID-19 virus test: no -negative on 08Dec2020. The patient died on the 30Dec2020 at 11:25 am in the morning. It was unknown if a postmortem was going to be carried out, after talking to the general practice surgery they advised that the general practitioner was only passed notification of the patient’s death that afternoon (04Jan2021). It was advised that they may go to the coroner but couldn’t give a definitive answer until the general practitioner had looked at the notification. It was not reported if an autopsy was performed. No follow up attempts are possible. No further information is expected.; Reported Cause(s) of Death: Death | |
| COVID19 VACCINE (COVID19) | possible myocardial infarction; Dyspnoea; unwell; Cough; This is a spontaneous report from a contactable physician downloaded from the Regulatory Agency. Regulatory authority GB-MHRA-WEBCOVID-20210105105739, other manufacturer number is GB-MHRA-ADR 24556743. A 76-year-old male patient received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE, Lot number: EJ0553-v0003), via unspecified route of administration on 19Dec2020 at single dose for COVID-19 vaccination. Medical history included diabetes mellitus, angiocardiogram, cardiac failure, hypertension, all from unspecified date and unknown if ongoing and cerebrovascular accident from 2001 and unknown if ongoing. Patient has not had symptoms associated with COVID-19 Patient has not been tested/or has had an inconclusive test for COVID-19. Unsure if patient is enrolled in clinical trial. Concomitant medication included amlodipine, acetylsalicylic acid (ASPIRIN (E.C.)), atorvastatin, bisoprolol, fluticasone propionate (FLIXONASE), folic acid, colecalciferol (FULTIUM-D3), furosemide, latanoprost, levothyroxine, insulin aspart (NOVORAPID), ramipril and insulin detemir (LEVEMIR). On 24Dec2020, the patient experienced a cough. It was noted that the patient’s son and wife had already been coughing but no coronavirus tests had been done at the time of this event. On an unknown date, the patient experienced dyspnoea. It was noted that the he had become increasingly short of breath and unwell. On 28Dec2020, the patient died. It was noted to be a possible myocardial infarction. The patients COVID test score was unknown. The autopsy was awaited at the time of this report. The outcome of the event possible myocardial infarction was fatal, while other events were unknown. No follow-up attempts are possible. No further information is expected.; Reported Cause(s) of Death: possible myocardial infarction | |
| COVID19 VACCINE (COVID19) | At night they found him lifeless. Probably following acute MI; pain in the arm and swelling in the arm of vaccination; pain in the arm and swelling in the arm of vaccination; This is a spontaneous report from a contactable other healthcare professional via Division of Health. The other healthcare professional reported similar events for three patients. This is the second of three reports. A male patient of an unspecified age received BNT162B2 (lot# EK4175), via an unspecified route of administration on 25Dec2020 at single dose for Covid-19 immunisation. Medical history included chronic obstructive pulmonary disease (COPD) with smoking background, atrial fibrillation, aortic stenosis, diabetes with damage to all target organs (nephropathy, retinopathy, neuropathy), carotid stenosis, deep vein thrombosis (DVT) history, history of alcohol use with hepatitis, history of Hodgkin’s lymphoma after successful chemotherapy treatment, got around on a scooter. The patient’s concomitant medications were not reported. The patient was vaccinated on 25Dec2020 and passed away at home on 28Dec2020. Before his death, according to his daughter, he complained about pain in the arm and swelling in the arm of vaccination on an unspecified date of Dec2020. At night they found him lifeless. Probably following acute myocardial infarction (MI). The outcome of pain in the arm and swelling in the arm of vaccination was unknown, acute MI was fatal. It was not reported if an autopsy was performed. Follow-up attempts are completed. No further information is expected.; Sender’s Comments: Fatal acute myocardial infarction is more likely attributed to the patient underlying medical conditions including vascular stenosis and diabetes with complications. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate,Linked Report(s) : IL-PFIZER INC-2020519349 same reporter/product, similar event, different patient;IL-PFIZER INC-2021009752 same reporter/product, similar event, different patient; Reported Cause(s) of Death: acute MI | |
| COVID19 VACCINE (COVID19) | SEPSIS; respiratory distress; PLEURAL EFFUSION; This is a spontaneous report received from other healthcare professional via the Division of epidemiology of the Ministry of Health. The other healthcare professional reported similar events for three patients. This is the third of three reports. A 91-year-old male patient received bnt162b2 (PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration on 30Dec2020 at single dose for covid-19 immunisation. Medical history included known background of blood pressure disease, diabetes, malignant bladder from an unknown date and unknown if ongoing. The patient’s concomitant medications were not reported. Patient was received at the emergency room 3 days after receiving the corona vaccine in Jan2021, with fever, vomiting more than 40 times, in respiratory distress, was hospitalized in internal medicine department with sepsis diagnosis due to respiratory distress and pleural effusion, intubated, his condition was serious, patient passed away on 04Jan2021. Cause of death was reported as sepsis, respiratory distress and pleural effusion. It was not reported if an autopsy was performed. Follow-up attempts are completed. No further information is expected. Information about batch/lot number cannot be obtained.; Sender’s Comments: Based on the information currently provided, the fatal events sepsis, respiratory distress and pleural effusion are more likely attributed to intercurrent infectious conditions associated with the advanced old patient underlying diseases . The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate.,Linked Report(s) : IL-PFIZER INC-2020519349 same reporter, product, similar event, different patient;IL-PFIZER INC-2021009751 same reporter, product, similar event, different patient; Reported Cause(s) of Death: SEPSIS; respiratory distress; PLEURAL EFFUSION | |
| COVID19 VACCINE (COVID19) | About 15 minutes after receiving the vaccine she felt palpitations. She was monitored for another 15 minutes and while she was walking to her car se started noticing sore throat associated with inability to talk, unable to swallow secretions, and swelling the lips. Patient presented to the emergency room where she received EpiPen dose. Received diphenhydramine, famotidine, and prednisone. Lip swelling and sore throat began improving in ED. | |
| COVID19 VACCINE (COVID19) | a left sided nystagmus; urgent MRI showing multiple brain lesions consistent with Acute disseminated encephalomyelitis; Patient developed paresthesias on entire right side of body / The paresthesias continued, not progressing; a brief headache; episode of dizziness / developed severe dizziness; This is a spontaneous report from a contactable physician (patient). A 34-year-old female patient received first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE, lot number: EK5730) intramuscularly at right arm on 21Dec2020 07:00 at single dose for COVID-19 immunization. Medical history included known allergies to sulfa meds. The patient’s concomitant medications included multivitamins [vitamins nos] within 2 weeks of vaccination. Patient developed paresthesias on entire right side of body after a brief headache and episode of dizziness, all since 29Dec2020 15: 00. The paresthesias continued, not progressing, but patient was advised to obtain an MRI Brain and C spine as an outpatient. On 02Jan2021, the patient developed severe dizziness and a left sided nystagmus. She went to the ER and underwent urgent MRI showing multiple brain lesions consistent with acute disseminated encephalomyelitis. Lumbar puncture (LP) was performed, awaiting final results. Patient was admitted and was receiving IV steroids (solumedrol). Duration of hospitalization was 5 days since Dec2020. Patient did not receive any other vaccines within 4 weeks prior to the COVID vaccine. Prior to vaccination, the patient was not diagnosed with COVID-19. The patient underwent lab tests and procedures, which included Nasal Swab and Rapid covid swab, both on 02Jan2020 with result of negative. The outcome of the events was recovering.; Sender’s Comments: Based on temporal association and lack of other provided etiology, a possible contributory role of suspect BNT162B2 vaccine cannot be excluded for reported acute disseminated encephalomyelitis, paraesthesia, headache, dizziness and nystagmus. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | Cp initially that resolved in seconds. Then severe muscle aches, fatigue, temp 1 week,excruciating joint pain continues now. Malaise. | |
| COVID19 VACCINE (COVID19) | difficulty breathing/swallowing; difficulty breathing/swallowing; Hives/hives all over including in her mouth; This is a spontaneous report from a contactable consumer (spouse). A 46-year-old female patient (wife) received BNT162B2 (Pfizer-Biontech Covid-19 Vaccine, lot number: EH9899) via unspecified route of administration at arm right on 29Dec2020 at single dose for Covid-19 vaccine. Medical history included ongoing anxiety diagnosed 22 years ago. Concomitant medications were not none. Patient received the vaccine and was one big hive (01Jan2021). She went to the ER on sat and will have to go back. Caller is asking where she should go. patient went through the VAERS report 3 times. She did not have any sides effects. patient is a frontline worker. On 29Dec2020 she got the Covid vaccine. On Sat 02Jan2021 she went the ER with hives all over including in her mouth, stated she had difficulty breathing/swallowing, was given medication to take home and discharged. She was readmitted yesterday 05Jan2020 with worsening symptoms and needed to be given a prednisone nebulizer. they had two ER visits. No Investigation Assessment. patient was not recovered from the event hives/hives all over including in her mouth, the final outcome of other events was unknown. | |
| COVID19 VACCINE (COVID19) | 3:07 pm lung sounds diminished oxygen sats 68%, oxygen applied Oxygen sats remained low for next 36 hours ( patient on Hospice care ) expired 6:22 am 1-8-21 | |
| COVID19 VACCINE (COVID19) | 1/6/21 Pt received vaccine and complained of difficulty swallowing and rapid heart rate. Pt received methylprednisolone 125mg IVP, diphenhydramine 25mg IVP, & famotidine 20mg IVP. Pt reported improvement and was discharged. Sent home on diphenhydramine and oral prednisone. 1/7/21 Pt unable to swallow her own secretions and experienced eyelid swelling. Pt vomitted. Pt received epinephrine and Benadryl X 1 dose each. Pt then transported to hospital via ambulance. Reason for admission – acute respiratory failure secondary to anaphylactic reaction. Decision was made to emergently intubate the patient for airway protection despite aggressive intervention. Pt successfully extubated 1/8/21. Plan to discharge home and start Medrol Dose Pack 1/9/21. | |
| COVID19 VACCINE (COVID19) | Swelling of lips & tongue, tightening of throat. Quivering of arms & legs. Tightening of chest. Dizzyness lightheaded. | |
| COVID19 VACCINE (COVID19) | Anaphylactic reaction 6 days post vaccine 24Dec2020; I had severe chest tightness; SOB; throat soreness; hoarse voice; mouth swelling; This is a spontaneous report from a contactable physician, the patient. A 34-year-old non-pregnant female patient received the first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 mRNA VACCINE; Lot Number: EL0140), via an unspecified route of administration in the left arm on 18Dec2020 at 15:30 (at the age of 34-years-old) as a single dose for COVID-19 immunization. Medical history included severe dust mite allergy (based on skin test). Prior to the vaccination, the patient was not diagnosed with COVID-19. Concomitant medications included cetirizine hydrochloride (MANUFACTURER UNKNOWN), hydrocodone bitartrate/paracetamol (NORCO), ibuprofen (MANUFACTURER UNKNOWN), and ondansetron (ZOFRAN); all for unspecified indications from unknown dates and unknown if ongoing. The patient did not receive any other vaccines within four weeks prior to the vaccination. On 24Dec2020 at 10:00, 6 days post vaccination, the patient experienced anaphylactic reaction, severe chest tightness, shortness of breath, throat soreness, hoarse voice, and mouth swelling; all reported as life threatening. The events led to an emergency room visit and she was given epinephrine (EPI-PEN), methylprednisolone (SOLUMEDROL), and diphenhydramine hydrochloride (BENADRYL) as treatment. The patient stated that she developed the reactions 45 minutes after she took premedications for a dilatation and curettage procedure. The premedications included ibuprofen, hydrocodone bitartrate/paracetamol, ondansetron. She stated she had taken these medications several times before and this was the first time she had this reaction. Since the vaccination, the patient had not been tested for COVID-19. The clinical outcomes of the anaphylactic reaction, severe chest tightness, shortness of breath, throat soreness, hoarse voice, and mouth swelling were recovered on unknown dates.; Sender’s Comments: Anaphylactic reactions presented as chest tightness, shortness of breath, throat soreness, hoarse voice, and mouth swelling, developed 45 minutes after premedications including included ibuprofen, hydrocodone bitartrate/paracetamol, ondansetron for a dilatation and curettage procedure and 6 days post vaccination with BNT162B2, the event therefore is most likely attributed to these premedications unrelated to the vaccine use. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | C/o shortness of breath routine oxygen increased cannula changed to mask oxygen sats at 88% | |
| COVID19 VACCINE (COVID19) | Labored breathing with oxygen running at 4l/min, muscle weakness | |
| COVID19 VACCINE (COVID19) | Fever to 103.7F, respiratory rate 36. Was transferred from facility to hospital. Since then has been found to have gram-negative rod bacteremia, although urinalysis was negative, urine culture pending. Patient has since defervesced after receiving 1 dose of cefepime. Overall the most likely cause of fever seems to be urosepsis w/ bacteremia, pending confirmation with urine & blood cultures. | |
| COVID19 VACCINE (COVID19) | The patient was found deceased at home about 24 hours after immunization. Date of Death:: 12/29/2020; estimated time of death 6:00pm | |
| COVID19 VACCINE (COVID19) | Patient received vaccine on 1/4/2021. He was in Hospice for CHF and renal failure, but was able to get up in his wheelchair and eat and take medications and talk. On 1/5/2021 am, he was noted to be very lethargic an could only mumble, could not swallow. No localizing neurologic findings. He was too lethargic to get up in chair. | |
| COVID19 VACCINE (COVID19) | initial; swelling of tongue, tingling and numbness in legs, syncope. later; HA | |
| COVID19 VACCINE (COVID19) | Resident displayed with confusion/shaking at 1400, condition worsens at time went. Resident unable to state where he is, knows his name. can tell you he does not feel right. Temp 97.3, p 88, O2 91%, Bp 214/116 Transferred to ED with fever, temp of 103, and shortness of breath, admitted to ICU Positive COVID-19 test at hospital. Diagnoses include acute COVID-19 pneumonia and hypoxia. PO had confusion, fatigue, weakness, hypoxia, increased BP | |
| COVID19 VACCINE (COVID19) | Swelling of throat and tongue, anaphylaxis, hives, redness, swelling | |
| COVID19 VACCINE (COVID19) | shoulder joint pain, injection was given in joint…. I am now on prednisone, physical therapy, if this doesnt help will need a MRI . | |
| COVID19 VACCINE (COVID19) | She began with an earache and dizziness. Pain got so severe that she could no longer take it. Went to the doctor which she was put on pain medications. Went to ER on 1/6 and on 1/7 went to her PCP. Still in severe pain. | |
| COVID19 VACCINE (COVID19) | Patient experiencing Chest pain and elevated troponin. Patient taken to the cath lab and treated for suspected stress induced cardiomyopathy. | |
| COVID19 VACCINE (COVID19) | ITP Plt 2 | |
| COVID19 VACCINE (COVID19) | Notified today that he passed away. No other details known at this time. | |
| COVID19 VACCINE (COVID19) | Hospital on 1/2 – then again on 1/5, transferred and admitted to hospital, discharged 1/6 Abnormal reflex/weakness back pain paresthesia and weakness of legs abdominal pain evaluation for possible GBS post covid 19 vaccine | |
| COVID19 VACCINE (COVID19) | 29-year-old previously healthy female presenting today with difficulty sleeping, sore throat, and nausea after receiving the second Pfizer COVID-19 vaccine around 10 AM. Patient says that after her first dose of the vaccine she had mild sore throat and hoarse voice that resolved spontaneously. She had her vaccine around 10, several hours later before coming to the emergency department between 2–230 she had the sudden onset of difficulty speaking with associated sore throat and nausea. She has had dry heaving but no large amounts of vomiting. She has not had stridor, wheezing, shortness of breath, syncope, or the development of a rash or hives. She has not had a reaction to her prior vaccines she does not have any other allergies in general. She has otherwise been well recently without infectious symptoms including fevers, chills, cough, and has not been exposed to Covid to her knowledge. Medications administered in ED included diphenhydramine 50 mg IV once, dexamethasone 10 mg IV once, famotidine 40 mg IV once, ondansetron 4 mg IV once. Had brief episode of shaking and R arm rigidity in the ED. Patient denies LOC during the episode, did not have a post ictal state, no tongue biting or episodes of incontinence. Given single event without LOC, less concern about episode being a seizure. Patient has had no further episodes of shaking since admission. CT brain was wnl, no FNDs noted on exam. | |
| COVID19 VACCINE (COVID19) | I am breastfeeding. My daughter had seizure like episodes starting on Saturday 1/2, Sunday 1/3, Monday, 1/4 and 2 times on Tuesday 1/5. | |
| COVID19 VACCINE (COVID19) | Patient received first dose of vaccine on 12/28, developed COVID-19 infection shortly thereafter and expired on 1/6/2021. | |
| COVID19 VACCINE (COVID19) | Patient received first dose of vaccine on 12/28, developed COVID-19 infection shortly thereafter and expired on 1/4/2021 | |
| COVID19 VACCINE (COVID19) | Cardiac event, 2 days after vaccination, patient expired. | |
| COVID19 VACCINE (COVID19) | Fever, shortness of breath and chest pain that resulted in a heart attack a few hours after vaccination | |
| COVID19 VACCINE (COVID19) | Acute allergic reaction Tongue swelling Facial swelling Throat swelling Rash on throat and chest Redness in throat Diaphoresis Momentary loss of consciousness | |
| COVID19 VACCINE (COVID19) | Altered Mental Status began the middle of the night of 01/06/21 and 01/07/21 with worsening overall status- definitive symptoms unknown to this reporter, this person was admitted to the local hospital at approximately 1800 01/07/2021. This reporter was not told the admitting diagnosis or any defining symptoms, only that the person was admitted. | |
| COVID19 VACCINE (COVID19) | Medical docter state patient has a acute cardiac attack | |
| COVID19 VACCINE (COVID19) | Initial itching at injection site, observed and returned to work. Came back ~30-40 minutes later with itchiness in throat and hives to arm. Given Benadryl PO and observed for extended period of time. Symptoms not resolving. Patient transferred to Emergency Department for further care. At that point observed to have full body rash, SOB. Given Epi while in ED. Developed tachycardia, hypotension. Treatment continued. | |
| COVID19 VACCINE (COVID19) | After about 15 min: hr went to over 170bpm , flushed sensation, brain fog, was driving at the time and my brain wouldn?t figure out how to call 911 , eventually I figured it out but it took extreme mental effort. Paramedics came and my pulse was From 100 – 170 changing rapidly, bp 150-175/x changing rapidly, symptoms would come and go about 8 times on way to hospital. In ER same thing then about 2 hrs go by and then all started again. Pulse remained at 90- 100bpm at least until 2am. (My resting hr is between 40 and 50, my normal bp is usually sub 120/80) I later realized my hands were swollen, mostly on my left side. Lingering symptoms include brain fog, tiredness, and headache. Maybe an exasperating issue was I had low potassium levels likely making symptoms worse – I worked out heavily early in the morning, probably had low intake of potassium that day and day before, and maybe slightly dehydrated from my workout – I do Functional lifting, run, and other types of exercise regularly. | |
| COVID19 VACCINE (COVID19) | Developed hypercapnic respiratory failure, CHF exacerbation – readmitted to Hospital. In ICU with BIPAP | |
| COVID19 VACCINE (COVID19) | patient begin to feel bad that night was admitted into hospital sometime in the next couple of days for dehydration, patient discharged home and then readmitted to hospital for positive covid testing after feeling very ill. | |
| COVID19 VACCINE (COVID19) | Patient presented to the emergency department with sensory loss and loss of reflexes, evaluated by neurology and diagnosed with Guillain- Barre Syndrome thought to be secondary to the Pfizer Covid Vaccine | |
| COVID19 VACCINE (COVID19) | Death | |
| COVID19 VACCINE (COVID19) | I had a myocardial infarction on December 27, 2020. I had received my first vaccination for COVID-19 on December 22, 2020. Not sure if these are related but I felt I should report it. | |
| COVID19 VACCINE (COVID19) | Low grade Fever, headache needing admission Intracranial hemorrhage with hypertension Medical management for hypertensive emergency Received surgical evacuation admitted in Intensive care, | |
| COVID19 VACCINE (COVID19) | 1/4/21: Headache, Dizziness & Fatigue 1/7/21: Left Sided Facial Droop | |
| COVID19 VACCINE (COVID19) | Shortness of breath, cough, rash on face and neck, arthralgia | |
| COVID19 VACCINE (COVID19) | Patient received COVID vaccination around 12:15pm. Patient was monitored for the appropriate amount of time by nursing staff. Patient passed away at 2:15pm. | |
| COVID19 VACCINE (COVID19) | Diarrhea followed by death 24 hrs after vaccination | |
| COVID19 VACCINE (COVID19) | Patient is a 32 yo G2P1001 with EDD 5/2/2021 by 7w US. She had the first dose of the Pfizer Covid 19 vaccination on 12/17/2020 at the Health Clinic and the second dose on 1/7/2021 at 1115 am. She began having abdominal pain and vaginal bleeding at 315 sm on 1/8/2021 progressing to a previable (22w2d) preterm birth at 739pm on 1/8/2021. | |
| COVID19 VACCINE (COVID19) | Pt experienced extreme fatigue and sleepiness the day following her second vaccination for Covid 19 and was found by her family after collapsing on 1/6/21 at 05:30. Upon arousal, she experienced headache, vomiting, weakness, difficulty speaking and difficulty walking with lower extremity weakness. She was taken to urgent care and subsequently admitted for evaluation at hospital and found to have a normal chemistry, blood count, normal lumbar puncture and normal imaging of her neck and brain. Discharge summary notes 3/5 strength and hyporeflexia throughout. Pt had televisit consult with psychiatry and neurology. She is subsequently to be discharged to a Facility without explanation for her sudden onset of progressive lower extremity and vocal weakness. She is noted to have a history of shellfish allergy. She experienced mild symptoms after the first vaccination, but no neurologic or vascular symptoms at that time. | |
| COVID19 VACCINE (COVID19) | I am a physician and I got dose 2 at 1:30pm on Jan 4. Next afternoon, Jan 5, I got severe myalgias, fever up to 100, severe fatigue, went home after work and slept til the next morning, went to work, took ibuprofen, and the myalgias improved and felt better. But around 3 pm, Jan 6, I got mild vertigo. By about 7pm Jan 6, I noticed my L ear didn’t hear well. I changed the battery in my hearing aide and cleaned it but It made no difference. I woke up on Jan 7 with severe vertigo and hearing loss. I did Epley’s maneuvers with no effect. I have had similar episodes. I went to work, but gave up when I could not hear patients talking to me. I went to the Emergency Dept and got admitted. I was too unsteady on my feet. Audiogram showed profound hearing loss both ears and almost complete loss of discrmination in R ear. I was put on high dose steroids. Also having tinnitus (mostly whooshing sound of my own pulse). MRI negative. Blood work negative. Some mild improvement now, after 1 dose steroids. | |
| COVID19 VACCINE (COVID19) | Fever up to 102.9, chills, headache, hypertension, tachycardia, dyspnea. | |
| COVID19 VACCINE (COVID19) | “Myocardial Infarction: patient began to complain of severe chest pain 3 hours after the vaccine was given .. Vaccine NDC # 59267-1000-1. 0.3 ml given by RN. Patient called his PCP: “”… I had very bad chest and shoulder pains, neck pains and slight fever from 9 pm until early this morning (Jan 8). My blood pressure was 155/95 mmHg. Should I see you today? Still feel sore all upper body. Above message received at 0720 am (Jan 8) and the patient was called back at 0757 am (Jan 8): patient was told that many of the side effects above were related to the vaccine but the chest pain was worrisome and the provider requested the patient go to the emergency room. Patient understood the importance to seek medical attention….. Emergency Room notes: seen by MD on Jan 9. Note at 0749: patient complained of chest pain on/off since received COVID vaccine on Jan 7. Pain was substernal and radiated to the left shoulder, assoc with some SOB. EKG obtained and revealed ST segment elevation and a “”cardiac alert”” was called.” | |
| COVID19 VACCINE (COVID19) | At around 11:45pm tingling started in my left eyebrow. I thought there was something in my eyebrow and after time of wiping it a few times I went to look into the mirror and realized that nothing was there. about and hour later I got a drink and a snack to eat and I realized I had a numb sensation in the left corner of my lip. As a nurse I went though the signs of a stroke with 2 other nurses I called. Everything was normal other than that sensation. Thinking I was just overly tired I went to bed. When I woke up the next day I felt okay until I went to drink some coffee and the numb sensation in my corner lip was still there. Now I was concerned and called employee health and was instructed to go to the ER to role out a stoke. I followed employee health’s instructions and went to the ER. I was diagnosed with facial paresthesia and discharged with instructions to come back if symptoms got worse. Symptoms persisted all day and around 7 I called my mom on video chat to show a visual facial twitch on the left side of my face. right after I got off the phone with her the left side of my face drooped and my fiance immediately drove me to the closest ER. I was seen immediately and they decided to admit me. The did labs and head CT. They admitted me to labor and delivery because there were no other rooms. The next day I was seen by a neurologist and many doctors. | |
| COVID19 VACCINE (COVID19) | Patient was unresponsive in her room during the night, had gotten the vaccine this morning, 911 called. Had right arm pain and loss of consciousness. EMS got 180/104 BP and blood glucose was 122. Was transported to hospital. Returned to the facility the next day with no complications, was just fatigued. | |
| COVID19 VACCINE (COVID19) | 8 hours after vaccine I experienced stomach pain and nausea. I then became very ill and extremely weak. I was laying on floor. Very difficult to talk and very dizzy. Unable to walk. Called 911 and went to ER. Had chest pressure and cardia work up was done. Dx wi ST elevation. | |
| COVID19 VACCINE (COVID19) | 7 day after site itching, hot swelling. Unsure if related 9 day after suffered CVA and have hyper coagulation | |
| COVID19 VACCINE (COVID19) | sudden sensorineural hearing loss in the right ear, audiology and ENT assessment, currently being treated with steroid medication | |
| COVID19 VACCINE (COVID19) | after receiving vaccine patient immediately felt warm, dizzy and started dry heaving. we dosed Zofran 4mg, gave one dose epi-pen, and 50mg Benadryl. patient still complained of chest pain and was dry heaving. she was then transported by ems to the hospital at 4pm. | |
| COVID19 VACCINE (COVID19) | Received vaccine 12/19/2020 at hospital around lunch time. Severe abd pain started 10 hours after vaccine administration with vomiting. I went to emergency room next morning Emergency appendectomy 12/20/2020. Had bleeding after surgery. Second surgery 12/21/2020 | |
| COVID19 VACCINE (COVID19) | Anaphylaxis | |
| COVID19 VACCINE (COVID19) | After the vaccine was administered I walked away maybe 50′ and I started to feel dizzy I felt light headed and as if I was drunk my legs feel real week they took me outside so I could catch some fridge fresh air and they set me down on a chair I was very dizzy my legs and my knees felt like I couldn’t stand up and they were very weak I kept seeing a the rails double vision and I started to have a tightness in the back of my neck I felt they warrant come over my head and my forehead got very very cold And then I felt as I was gonna blackout and pass out and I was gasping for air and suddenly my tongue went into a spasm and it went to the top of my the roof of my Roof of my mouth and I couldn’t breathe and I was able to send a message for someone to come and help me as I was sitting there by myself they rushed over by now looking at my text message it was for 02 which was within 15 minutes of the vaccine when I had my 1st episode and then minutes after that 3 more came with the same oh unable to swallow I lost the ability to swallow and my tongue fell like I had no control it was just automatically stuck to the roof of my mouth.. Upon the arrival of Ems I was told there was no treatment and there was nothing they could do told me to wait 24 to 48 hours in the symptoms should subside it’s been over 72 hours in the symptoms are still occurring. I continue to feel dizzy light headed and now have high blood pressure which was not present before visit ER prescriptions for steroids with issued, I Told to go home and rest. Followed up with family doctor in the morning and was told it was not an allergic anaphylactic reaction probably more so neurologically ransom blood tests waiting for results continue to have loss of control over tounge spasms unable to eat Accompanied by fatigue dizziness and high blood pressure | |
| COVID19 VACCINE (COVID19) | On 01/07/2021 I woke up at 0300am with chills, headache, body aches, joint pain, fever of 101.2 and swollen left axillary lymph nodes. I took Tylenol and Benadryl and it relieved the fever/headache/body aches/joint pain, however the lymph nodes in my left axillary remained swollen. I continue to take Tylenol for the fever/body aches/pains without relief for the swollen and painful axillary lymph nodes. Warm compresses do help to relieve the pain temporarily but they remain painfully swollen. On 01/08/21 I called my doctors office to ask if it was normal to experience such painfully swollen axillary lymph nodes to which they stated ?we don?t know, it is too soon for us to tell what?s normal and what isn?t normal right now.? They did not offer any suggestions to relieve the pain or swelling. The morning of 01/09/2021 , I called Employee Health at my hospital (my place of work and also where I received the vaccine) and they also stated they didn?t know if this was a normal reaction due to the newness of the vaccine. A couple hours later, employee health emailed me a link to the VAERS reporting website and asked me to file a report. | |
| COVID19 VACCINE (COVID19) | I am currently breastfeeding my 5-month-old son. I received my first vaccine on 12/28/2020 and directly breastfed within 4 hours of receiving the vaccine. Two days after my vaccine my son was at daycare and had two large diarrhea blowouts and two large emeses followed by a 1-minute episode where he was limp with entire body cyanosis and in-and-out of consciousness. He also had a maculopapular rash on his torso. EMS was called. He was observed in the emergency department for a few hours then recovered well without intervention and did not require hospitalization. EKG was normal. He has continued to be well and back to baseline since the event. | |
| COVID19 VACCINE (COVID19) | “Dizziness started within 30 minutes after injection on 01/08/21 and felt “”off””. On 01/09/21 approx. 0800 Patient began feeling body aches, fevers, injection site pain, increased dizziness, and nausea. At approximately 1pm patient began vomiting and having diarrhea. Symptoms worsened over the next couple hours to where patient was unable to walk without stumbling. Wife witnessed patient becoming very pale and almost pass out at approx. 5:30pm. Patient states he feels like he’s in slow motion. Patient is unable to maintain balance when walking and reports increasing fatigue and weakness.” | |
| COVID19 VACCINE (COVID19) | Started severe belly pain and went to Emergency room and diagnosed with mesenteric vein thrombosis after the CT scan of the abdomen, treated with heparin drip, antibiotic and discharged with anticoagulant pills(Eliquis). I am not sure that it is because of the vaccine my doctors are also not sure about it, but I am sure that I am a healthy person without any health issues . I am working as registered nurse, our unit is for covid-19 patient’s since march 2020 and I had covid -19 on August month and recovered after 3 weeks. | |
| COVID19 VACCINE (COVID19) | Pfizer-BioNTech COVID-19 Vaccine EUA Miscarriage – (date of vaccination 1/6/21, miscarriage symptoms (cramping) started 1/8/21, confirmed 1/10/21; estimated date of delivery 8/30/21) | |
| COVID19 VACCINE (COVID19) | Patient came into the emergency department on 1/8/21 with an acute ischemic stroke with complete occlusion of her left MCA. She had acute and complete flaccid paresis of her right face, arm, and leg, complete aphasia, and neglect of the right side of her body. NIHSS of 27. Onset of deficit was between 6:30pm-7:10pm. She recieved her 1st COVID-19 vaccine dose that morning at 10:31am. |
| COVID19 VACCINE (COVID19) | I was injected high on my shoulder, significantly higher than I?ve ever been injected in my life. I believe I have SIRVA. The pain has become so severe that I cannot use my left arm. The pain is intolerable. I take four Advil every six hours, ice my arm regularly, and keep my arm in a sling. The pain has gotten significantly worse with time (not better). I?ve never experienced pain like this from a vaccine in my life. No history of bursitis or shoulder injury. Again, the pain gets worse with time. I?m almost 48 hours post injection | |
|---|---|---|
| COVID19 VACCINE (COVID19) | 1/7-21 – Received second dose of pfizer covid-19 vaccine 1/8/21 – Fever, dizziness, headache 1/10/21 0250 was found not breathing. EMS performed CPR and patient deceased | |
| COVID19 VACCINE (COVID19) | The patient presented with left eye peripheral visual loss, left upper and lower extremity and facial numbness sensation and weakness. This started 1 hour after receiving COVID-19 vaccine at her place of employment. Pt was brought to CRMC via EMS. | |
| COVID19 VACCINE (COVID19) | Facial (cheek) numbness and swelling with slight face droop Swelling continued on 1/7/2021 On 1/8/2021, lip swelling and numbness and tongue numbness By 1/9/2021 4pm, swelling and numbness resolved but chills and muscle aches began | |
| COVID19 VACCINE (COVID19) | Patient received the Moderna Vaccine 1/2/21 at his VA Clinic. He received the vaccine that morning and by the evening he was not feeling well. He developed cough, weakness and fever and now is unable to ambulate. He has since been hospitalized and has tested positive for COVID-19. | |
| COVID19 VACCINE (COVID19) | I am not sure if related on not. This event was 13 days after my COVID-19 1/2 immunization. Otherwise, I am a very healthy physician, normal BMI, I have also been tested 5-6 times negative for COVID. I do get exposed in my job, but wear proper PPE. Viral infection in FEB that was like COVID-19 sx, I did AB test as soon as it was available, and negative. —The Event: Monday morning (1/4/21), after getting out of shower, I was talking to my husband (who is MD)and started having BROCA’s aphasia sx (could not get words out coherently), then fell into bed and started right wrist and right foot posturing. This lasted 10 min. I have non-memory of it, but my MD husband witnessed it. After 10 minutes, I was back to normal, except shaky and some word finding difficulties. After 30 min, totally back to normal. | |
| COVID19 VACCINE (COVID19) | pt received Moderna vaccine. next day he had high fevers up to 103, confusion. admitted to hospital. infectious work up negative. improved off antibiotics. | |
| COVID19 VACCINE (COVID19) | Resident appeared to be jaundice with yellow skin and eyes. Resident also complained of not feeling well. Urine was dark yellow. Resident short of breath. Resident was admitted to hospital and diagnosed with post-covid pneumonia. | |
| COVID19 VACCINE (COVID19) | Throat closing Pruritic throat and tongue Tingling lips and tongue Throat clearing Hoarse voice | |
| COVID19 VACCINE (COVID19) | Acute ischemic stroke, basilar occlusion | |
| COVID19 VACCINE (COVID19) | SOB | |
| COVID19 VACCINE (COVID19) | Nausea/vomiting and diarrhea loss of appetite tachicardia sent to er for hydration. | |
| COVID19 VACCINE (COVID19) | I immediately felt dizzy, and there was ringing in my ears, I felt faint. I also felt shakey. I have had these symptoms for 12 days, unchanged. I cannot work because of these symptoms. I have been to urgent care and have had lab work and an EKG | |
| COVID19 VACCINE (COVID19) | RECIEVED VACCINE 1/8/21 EXPIRED UNEXPECTED 1/10/21, NO ADVERSE REACTIONS NOTED | |
| COVID19 VACCINE (COVID19) | Two days following the first COVID vaccination he developed epigastric pain and was evaluated in the ER and was admitted for gallbladder surgery. He has a 6 year history of gallbladder disease but was not experiencing symptoms until after the COVID vaccination. | |
| COVID19 VACCINE (COVID19) | The patient had an apparent cardiac arrest on 12/23/20 and was admitted to the ICU. He was taken off of life support on 12/30/20. He had known cardiac disease. | |
| COVID19 VACCINE (COVID19) | Severe thrombocytopenia (plts 3k/uL), oral mucosal bleeding, bruising | |
| COVID19 VACCINE (COVID19) | Sudden, severe worst headache of her life, coupled with onset of oral herpetic lesions and inflammation to unrelated body part (belly button piercing) occurred one week after vaccination received. She presented to ED 12/28 noted to have hypokalemia and head CT showed mild occipital encephalitis, admitted overnight for obs subsequent brain MRI was normal. She was seen in my clinic 2 days later (1/4/21) and was started on 3 day course of Decadron, topical acyclovir for herpetic lesion. As she is a nurse, I kept her off work until resolution of symptoms. Seen again on 1/8/21, headache resolved. She did discuss CT and MRI results with neurologist. He was not convinced this was vaccine related. She is having 2nd dose of vaccine on 1/11/21. | |
| COVID19 VACCINE (COVID19) | Patient died, I have a copy of his vaccination card | |
| COVID19 VACCINE (COVID19) | Moderna vaccine dose #1 received in right shoulder on 12/31/20 at 2:15PM. Injection was uneventful other than sensation of pressure. I did notice at the time, but could not fully see, that the injection appeared to be much higher on the shoulder than normal. I immediately came home afterwards and relaxed. Approximately 2hrs later, I began experiencing extreme pain in my right shoulder. As the night progressed, the pain worsened to 10/10 with inability to move my arm. Later that night after requiring assistance to take off my shirt, I noticed that the bandaid overlying the injection site was very high, immediately below and bordering the acromion process. This was concerning but I was hopeful the pain would go away over the next few days. I took tylenol that night. I woke up multiple times during the night because the pain was so severe. When I woke up, the pain and inability to move my arm were still present. I began taking 800mg ibuprofen and 1000mg tylenol alternating Q4 throughout the next few days. The pain and disability remained so severe that I required assistance performing ADLs for the next 4 days. On day four, with the pain not resolved and still severe despite consistent advil and tylenol use, I began having concern for shoulder injury related to vaccine administration. The next day I decided to seek an evaluation by an orthopedist. I am a physician and was unable to perform basic tasks and ADLs. I happened to have a few days off after the injection but would not have been able to work had I not. After an evaluation by the an orthopedic PA, I obtained an MRI of my right shoulder with results shown below – as I expected evidence of rotator cuff tendinopathy and subdeltoid bursitis consistent with SIRVA. As of now, I still have limited range of motion and shoulder pain on the right which has improved slightly but is far from resolved. I still have difficulty with certain ADLs including putting clothes on and off, lifting items, and tasks that require raising my right arm above my head. I am concerned with the possibility of long term and/or permanent damage after reviewing the literature. I am also concerned about how many other healthcare workers the person who gave me my vaccine may be injuring and/or causing permanent harm to. | |
| COVID19 VACCINE (COVID19) | Pain at site of injection, eyes, throat, face swelling. Unclear thinking, hoarse speech, headache, hives, swelling. Intervention taken immediately. Ongoing 11 days: SOB, headaches, nose bleeds, coughing, blood sugars triple, hair falling out, major swelling, dizziness. | |
| COVID19 VACCINE (COVID19) | Death within 24 hours after dose; This is a spontaneous report from a contactable consumer downloaded from the regulatory authority (GB-MHRA-EYC 00236003 and GB-MHRA-ADR 24545815). A 78-year-old male patient received BNT162B2 (COMIRNATY), via an unspecified route of administration, on 20Dec2020 at 16:00 as a single dose for COVID-19 immunization. Medical history included cardiac disease and lung disease. The patient had no known allergies. Concomitant medications included an unspecified hypertensive taken for hypertension, an unspecified drug for ischaemic heart disease, and an unspecified drug for chronic obstructive pulmonary disease (COPD). The patient experienced death within 24 hours after dose on 21Dec2020. The event was reported as fatal. The clinical course was reported as follows: The patient was observed for 15 minutes after the dose was given and had no side effects. In the evening, the patient felt well. The patient received the vaccination as he was a high risk patient, elderly, and with a background of cardiac and lung disease. The clinical outcome of death within 24 hours after dose was fatal. The patient died on 21Dec2020. The cause of death was unexplained. It was unknown if an autopsy was performed. The reporter assessed the causality between the vaccination and death as unlikely. No follow-up attempts possible; information on lot and batch numbers cannot be obtained.; Reported Cause(s) of Death: Death unexplained | |
| COVID19 VACCINE (COVID19) | Death; Head ache and dizziness; Head ache and dizziness; Spitting blood; Vomiting blood; Nose bleed; This is a spontaneous report a contactable consumer downloaded from the Regulatory Authority(GB-MHRA-WEBCOVID-20201220100831 and GB-MHRA-ADR 24545199). A male patient of an unspecified age received BNT162B2 (COMIRNATY), via an unspecified route of administration, on 17Dec2020 as a single dose for COVID-19 immunisation. Medical history included vitamin D3 deficiency and asthma. Concomitant medications included colecalciferol (MANUFACTURER UNKNOWN) for vitamin deficiency and salbutamol sulfate (VENTOLINE) for asthma. The patient experienced nose bleed on 17Dec2020, head ache and dizziness, spitting blood, and vomiting blood on 18Dec2020, and death on 20Dec2020. All of the events were reported as fatal. It was reported that a healthcare professional advised the patient to take unspecified pain medication after explaining mild and strong side effects to help with pain. The patient underwent lab tests and procedures which included COVID-19 virus test: No – negative COVID-19 test on an unspecified date. Therapeutic measures were taken as a result of nose bleed, head ache and dizziness, spitting blood, and vomiting blood as aforementioned. The clinical outcome of nose bleed, head ache and dizziness, spitting blood, vomiting blood, and death was fatal. The patient died on 20Dec2020. The cause of death was unexplained. It was not reported if an autopsy was performed. It was also reported that since the vaccination, the patient had not been tested positive for COVID-19. No follow-up attempts are possible; information about lot/batch number cannot be obtained.; Reported Cause(s) of Death: Death unexplained | |
| COVID19 VACCINE (COVID19) | Complete loss of smell and taste; Complete loss of smell and taste; This is a spontaneous report from a contactable physician reported for herself. A 37-year-old female patient received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE; brand: PfizerbioNtech, lot number: EH9888) intramuscularly at left arm on 17Dec2020 at 03:00 PM at a single dose (dose number: 1) for COVID-19 immunization. Medical history was reported as none. No known allergies (no allergies to medications, food, or other products). The patient was not pregnant. The patient did not receive any other vaccines within 4 weeks prior to the COVID vaccine. No other medications the patient received within 2 weeks of vaccination. Facility where the most recent COVID-19 vaccine was administered was at hospital. The patient experienced adverse event complete loss of smell and taste on 19Dec2020 at 07:00 AM. The event was considered as serious due to resulted in disability or permanent damage. No treatment received for the event. The outcome of event was not resolved. Prior to vaccination, the patient was not diagnosed with COVID-19. Since the vaccination, the patient had not been tested for COVID-19.; Sender’s Comments: Based on the current available information and the plausible drug-event temporal association, a possible contributory role of the suspect product BNT162B2 to the development of events complete loss of smell and taste cannot be totally excluded. The case will be reassessed if additional information becomes available. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | encephalitis; This is a spontaneous report from a contactable consumer. A patient of unspecified age and gender received BNT162B2(PFIZER-BIONTECH COVID-19 VACCINE) , via an unspecified route of administration on an unspecified date at single dose for COVID-19 immunization. The patient was allergic to UTI(urinary tract infection) infection medication (i.e. sulfamethoxazole, trimethoprim etc.). Concomitant medications were not reported. The patient got encephalitis and was put in the ICU(intensive care unit) after getting vaccinated, led to hospitalization. Outcome of event was unknown. Information about lot/batch number has been requested. | |
| COVID19 VACCINE (COVID19) | Anaphylactic reaction | |
| COVID19 VACCINE (COVID19) | Pronounced dead 1/9/2021 at 12:42. Received first dose of vaccine 1/8/2021 | |
| COVID19 VACCINE (COVID19) | “Staff member checked on her at 3am and patient stated that she felt like she couldn’t breathe. 911 was called and taken to the hospital. While in the ambulance, patient coded. Patient was given CPR and “”brought back””. Once at the hospital, patient was placed on a ventilator and efforts were made to contact the guardian for end of life decisions. Two EEGs were given to determine that patient had no brain activity. Guardian, made the decision to end all life saving measures. Patient was taken off the ventilator on 1/9/2021 and passed away at 1:30am on 1/10/2021. The initial indication from the ICU doctor was the patient had a mucus plug that she couldn’t clear.” | |
| COVID19 VACCINE (COVID19) | Aprox 5 minutes after vaccine was given, patient stated he was not feeling well, asked for water and stated he was having trouble breathing. Was then assisted to clinic treatment room, vitals were taken: 08:45 B/P 171/110, HR 84, R 28 O2 97. EMS was activated. At 0900 158/94 HR 84 R 24 O2 97. Patient then voice he was having tightness in his throat. Epi Pen 0.3mg administered at 0900. At 0904 EMS arrived and patient taken to the nearest hospital. | |
| COVID19 VACCINE (COVID19) | Numbness and tingling bil upper extremities, seizure, temporary paralysis R arm. Started day after vaccine given, was observed overnight in hospital. | |
| COVID19 VACCINE (COVID19) | “Manager was notified that the employee had suffered a stoke-like “”serious medical event”” hours after the end of her shift, while at home.” | |
| COVID19 VACCINE (COVID19) | Patient had vaccine in Left arm. That same night patient had temp of 100.1 and the right neck at base of head to shoulder began to hurt patient was unable to swallow without pain in the next few days. Patient went to ER and was hospitalized for 2 days treated with IV steroids and 2 antibiotics (clindamycin and acyclovir). Patients symptoms resolved and patient was discharged without additional issues. The admitting physician was unable to identify the cause of these symptoms, but the vaccine could not be ruled out. | |
| COVID19 VACCINE (COVID19) | “1-2-2021 10:30 PM Complained Right arm/back hurt – took Tylenol 1-3-2021 Complained Right arm hurt, dizzy 1-4-2021 Felt better – did laundry, daughter found her deceased at 3:30 pm. Dr. at hospital said it was “”cardiac event”” according to death certificate.” | |
| COVID19 VACCINE (COVID19) | Upon assessment resident noted to have increased respirations, lungs CTA. Resident c/o increased fatigue and muscle aches. VS 202/180, 118, 22, 97.1, 96%. | |
| COVID19 VACCINE (COVID19) | Sever thrombocytopenia (platelet count 2,000) 8 days following Moderna COVID vaccine. Clinically suspicious for ITP. | |
| COVID19 VACCINE (COVID19) | Received vaccine on 1/5, began having swelling in bilateral hands and lower extremities on 1/7, along with fatigue. On 1/8 she reports new swelling started in her face (eyes and cheeks). Swelling has not improved today and fatigue has worsened. | |
| COVID19 VACCINE (COVID19) | Found on floor by CNA at 6 am. On assessment by charge nurse, VS: 99.6-94-16 212/105 manual. Noted increased confusion. Temp. 99.6 FBS 114. Resident had been provided tylenol just prior in shift for general discomfort (sore muscles) and low grade temp. On call for Dr notified of all and received order to send to ER. | |
| COVID19 VACCINE (COVID19) | Staff reported that patient was found Friday morning (Jan 8) sitting at a table with his head tilted forward and unresponsive to verbal or physical stimuli. Staff lowered patient to floor and started CPR. EMS was called and continued CPR at scene, however they were not able to revive patient. Patient was pronounced dead at the scene. Staff written statements following the death of patient show that he had a fall about 1 hr. prior. It is unknown if this fall contributed to patient’s death. An autopsy has been requested. | |
| COVID19 VACCINE (COVID19) | Acute anterior MI with death | |
| COVID19 VACCINE (COVID19) | Approximately 15 minutes after receiving the vaccine, client started c/o itching to face and lips. Transported to triage area. Where she started c/o burning lips also. vital signs monitored, Benadryl 50mg po given. In a few minutes she reported chest tightness, labored breathing. 1:44pm Epinephrine was given IM. client was on 02 increased to 15 Liters. She admits to decreased chest tightness and itching of face improving. She was taken by ambulance to hospital, where she was admitted overnight for observation. She was given Benadryl and epi X2 more time, once in ambulance and once at the hospital. She denies any problems since then. She did say that approx 3 weeks ago she was a contact to a case of covi | |
| COVID19 VACCINE (COVID19) | Patient is a 41 y.o. female who presented to the ED with complaints of a reaction after she took the COVID vaccine. Patient is an RN here and had earlier received COVID-19 vaccination (Moderna) around 0130 this afternoon. Soon after she experienced rash which was burning and itchy on her arms thighs and back. She reported to Occ health and was directed to the ED. While in the ED she experienced throat tightness and nausea. She had one episode of diarrhea. No tongue, lip swelling, SOB or difficulty swallowing. Denies any chest pain, palpitations, pre-syncope/syncope. No vomiting abdominal pain. H/o of allergy to fish mix and dust mite. | |
| COVID19 VACCINE (COVID19) | 01/06/21 at 6 pm, body aches, and chills 01/07/21 at 12am T102.2, SPO2 62% on room air. Was sent to ER and returned. 01/08/21 at SPO@ less then 60% on room air, non responsive to verbal tactile stimuli. Responsive to sternal rub only. Was sent to ER and admitted to ICU. | |
| COVID19 VACCINE (COVID19) | The resident resides in an independent living facility/apartment. The reporter at the center was informed by his daughter he was not feeling well on 1/1/2021 (specific symptoms could not be ascertained). He reportedly went to be COVID tested on 1/1/2020 and observed to be deceased in his apartment on 1/2/2020. I do not have confirmation of his COVID results, although the reporter indicates his daughter reports his test was positive. | |
| COVID19 VACCINE (COVID19) | Patient went to bed around 11pm on Saturday PM and sometime between then and 1:30am on Sunday morning got up and went into the living room without waking up her husband (which is normal). At 1:30am, the husband got up to use the restroom and she was out of bed then, but the husband did not know if she was having any problems at this time. When he got up at 7:45am, she was in the recliner and did not move or anything, which is normal for her. At 8:45am, the husband went back into the living room and tried to wake his wife and that is when he noticed there was no pulse and he called 9-1-1 at this time. EMS got on scene and did CPR for 30 mins and she was pronounced dead at 9:21am. | |
| COVID19 VACCINE (COVID19) | Approximately 10 minutes after receiving the vaccine she started experiencing numbness and inability to move all 4 extremities. No difficulty breathing or swallowing. Benedryl 25 mg was administered with no relief. EMS was called, she was transported to the ED and admitted to the hospital for evaluation. She was given Ativan 0.5 mg IV with some mild improvement in symptoms. She has had gradual improvement in her symptoms, now able to move her arms normally. She has persistent weakness and discomfort in both lower extremities and is unable to ambulate without assistance. | |
| COVID19 VACCINE (COVID19) | 1/8/2021 tachycardia Pulse 140, Fever T99.4 then 100.2 Lethargy, Somewhat Altered Mental status | |
| COVID19 VACCINE (COVID19) | patient died after collapsing in his home several hours after he received the vaccine; patient died after collapsing in his home several hours after he received the vaccine; The initial case was missing the following minimum criteria: the reporter does not have first-hand knowledge of the reported events and was not identifiable. Upon receipt of follow-up information on 06Jan2021, this case now contains all required information to be considered valid. This is a spontaneous report from a contactable healthcare professional via regulatory Authority. The regulatory authority reported similar events for three patients. This is the first of three reports. An 88-year-old male patient received BNT162B2 (PFIZER-BIONTECH COVID-19 mRNA VACCINE; Lot Number: EK4237), via an unspecified route of administration on 28Dec2020 as a single dose for COVID-19 immunization. Medical history included dementia, cardiac background with pacemaker, atrial fibrillation, heart failure, and penicillin allergy. The patient was not allergic to polyethylene glycol. The patient’s concomitant medications were not reported. On 29Dec2020, the patient died after collapsing in his home several hours after he received the vaccine. Outcome of collapsing was not recovered. The patient had no pulse when he arrived at the hospital. It was not reported if an autopsy was performed. The cause of death was unknown. Follow-up attempts are completed. No further information is expected.; Sender’s Comments: The advance old patient had underlying cardiac background with pacemaker, atrial fibrillation and heart failure, therefore the pre-existing cardiovascular medical conditions more likely provide explanations for collapsing lead to the patient death. More information especially death cause and autopsy results are needed for further meaningful assessment. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate.,Linked Report(s) : IL-PFIZER INC-2021009752 same reporter, product, similar event, different patient;IL-PFIZER INC-2021009751 same reporter, product, similar event, different patient; Reported Cause(s) of Death: Unknown cause of death | |
| COVID19 VACCINE (COVID19) | “Her voice became raspy, she could hardly talk, she could barely talk; her hand and whole arm swelled up; her hand and whole arm swelled up; Trouble breathing; This is a spontaneous report from a contactable healthcare professional (patient). A 57-year-old female patient received the 1st dose of bnt162b2 (BNT162B2, Manufacturer Pfizer-BioNTech) (lot# EK9231, exp date Apr2021), via an unspecified route of administration in the right arm, on 29Dec2020, at single dose, for COVID-19 immunization and diphenhydramine hydrochloride (BENADRYL), via an unspecified route of administration, at unknown posology, on 29Dec2020 (20 minutes prior to taking bnt162b2), for anaphylactic reaction. Medical history included ongoing thyroid disorder. Concomitant medications included apixaban (ELIQUIS), diltiazem (unknown trade name), losartan (unknown manufacturer), rosuvastatin calcium (CRESTOR), levothyroxine (unknown trade name), ongoing levothyroxine sodium (SYNTHROID) for thyroid disorder and metformin (unknown trade name). Previously the patient received unspecified influenza vaccine (reported as flu shot) for immunisation, on unspecified date, and experienced anaphylactic reaction treated with Benadryl. On 29Dec2020, an hour after getting the vaccine, the patient experienced her voice became raspy, she could hardly talk, she could barely talk with outcome of unknown, her hand and whole arm swelled up with outcome of unknown, trouble breathing with outcome of unknown. The reporter stated that her voice and everything reacted and made her go to the emergency room on 29Dec2020. She reported that had adverse effect more than normal. The event “”Her voice became raspy, she could hardly talk, she could barely talk”” caused patient’s hospitalization on unknown date. The action taken as a result of the events with diphenhydramine hydrochloride was post-therapy. Therapeutic measures were taken at the emergency room as a result of the events and included treatment with Topcid and Benadryl every 6 hours, 2x 40 mg of steroid. The information on the lot/batch number has been requested. Follow-up (02Jan2021): New information received from the same contactable healthcare professional reporting for herself includes: patient’s details, medical history, events updated, vaccine lot# and exp date, concomitant, seriousness of event “”Her voice became raspy, she could hardly talk, she could barely talk”” added as hospitalization, historical vaccine, suspect Benadryl details, therapeutic measures updated.; Sender’s Comments: A causal association between BNT162B2 and the reported events cannot be excluded based on a compatible temporal relation between vaccination and onset of events. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to RAs, Ethics Committees, and Investigators, as appropriate.” | |
| COVID19 VACCINE (COVID19) | Resident died suddenly and expectantly on 01/05/2021 | |
| COVID19 VACCINE (COVID19) | Patient received COVID-19 (Moderna) vaccine from the Health Department on afternoon of January 8, 2021 and went to sleep approximately 2300 that night. Was found unresponsive in bed the following morning and pronounced dead at 1336 on January 9, 2021 | |
| COVID19 VACCINE (COVID19) | Chills Hip pain | |
| COVID19 VACCINE (COVID19) | Resident had seizure like activity followed by a vagel response with large bowel movement. Resident then began to show signs of blood clot to left lower extremity. No pedal pulse, area on leg warm to touch. Left lower leg now cold to touch, stiff, purple and white in color. No other signs of modeling, body warm to touch, no fever noted. Respirations and pulse increased with low oxygen levels. Resident not responding to stimuli. | |
| COVID19 VACCINE (COVID19) | 38-year-old female who is healthcare worker and received first dose of COVID vaccine (Pfizer). Immediately after receiving the vaccine, patient developed lightheadedness, flushing, hives, wheezing and throat swelling. Patient was treated in an emergency department with epinephrine, gradually improved and was able to be sent home with an EpiPen, prednisone, hydroxyzine, and famotidine. The next day, patient again developed shortness of breath and her husband administered the EpiPen. EMS arrived and gave another dose of IM epinephrine and IV diphenhydramine. On arrival to the emergency department, the patient was altered, diaphoretic, tachypneic, tachycardic, and stridulous. Patient was given multiple doses of IM epinephrine and started on epinephrine drip. Stridor continued and was unresponsive to nebulized albuterol. Patient was then intubated and placed on mechanical ventilation. Other treatments included solumedrol, pepcid, magnesium sulfate, nebulized epinephrine, and IV fluids. admitted to the intensive care unit, weaned off epinephrine drip, and extubated the next day. Patient was monitored on hospital floor for one additional day and was then discharged with no residual symptoms. | |
| COVID19 VACCINE (COVID19) | Death in connection with the vaccination and/or Covid19 disease / positivity; Death in connection with the vaccination and/or Covid19 disease / positivity; This is a spontaneous report from a contactable physician. This physician reported similar events for two patients. This is the second of two reports. A patient of unspecified age and gender received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration on an unspecified date at single dose for COVID-19 immunization. The patient’s medical history and concomitant medications were not reported. It was reported 2 deceased were autopsied, death in connection with the vaccination or Covid19 disease / positivity.; Sender’s Comments: The information currently provided is too limited to make a meaningful medical assessment hence, the events are conservatively assessed as related to the suspect drug BNT162B2 until further information becomes available. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to RAs, Ethics Committees, and Investigators, as appropriate.,Linked Report(s) : DE-PFIZER INC-2021006738 same reporter, same product, same event, different patient; Reported Cause(s) of Death: Death in connection with the vaccination and/or Covid19 disease / positivity; Death in connection with the vaccination and/or Covid19 disease / positivity | |
| COVID19 VACCINE (COVID19) | “Sudden death; This is a spontaneous report downloaded from the regulatory authority DK-DKMA-WBS-0028211. Report was received from a contactable nurse via regulatory authority. A 94-years-old female patient received BNT162B2 (COMIRNATY) (Lot # EJ6797, exp date 30Apr2021), via intramuscular on 30Dec2020 at first single dose for covid-19 immunization. Medical history included ongoing periodic obstipation (periodic treated), ongoing dementia, ongoing atrial fibrillation, ongoing depression, tibia fracture from 01Nov2020 (treated conservatively). Concomitant medication included apixaban (ELIQUIS) from 09Apr2018 to unknown date for atrial fibrillation, sertraline from 20Jun2018 to unknown date for depression. The patient had not received BNT162B2 before. The patient experienced sudden death on 01Jan2021. There was no immediate illness until the time of vaccination and the nurse described that she “”seemed like herself and fresh””. The ADRs were by the reporter reported as fatal. Reported cause of death: Unknown caused of death, sudden death. No treatment due to the death was described. There is no information regarding test results. An autopsy was not performed. Causality: The doctor who issued the death certificate does not suspect that it is the COVID-19 vaccine that is the cause of her death. She slept quietly in, and was old. Due to reporting obligation this case is reported. If the regulatory authority receives supplemental significant information regarding this case the case will be re-submitted.; Reported Cause(s) of Death: Sudden death; Unknown cause of death” | |
| COVID19 VACCINE (COVID19) | Hypoxic respiratory failure; Dyspnea exacerbated; This is a spontaneous report downloaded from the Medicines Agency (MA) WEB DK-DKMA-WBS-0028232. The report was received from a contactable physician via The Medicines Agency (MA). A 45-year-old male patient received BNT162B2 (COMIRNATY) (Lot #: EJ6797, Expiration Date: 30Apr2021), via intramuscular on 30Dec2020 at single dose for Covid-19 vaccination. Medical history included ongoing treatment noncompliance, ongoing alcohol abuse chronic, ongoing psychosis, dyspnoea from 20Dec2020 and ongoing, ongoing hallucination, ongoing tobacco abuse, ongoing paranoid schizophrenia, chronic obstructive airways disease exacerbated from Aug2020 and ongoing, chronic obstructive airways disease exacerbated from Nov2020 to an unknown date (not ongoing), hypoxic down to 60 % from 20Dec2020 and ongoing, Amphetamine abuse (not ongoing), ongoing pain, ongoing opioid abuse, ongoing anxiety, and ongoing insomnia. There is no information regarding past medication. Concomitant medication included prednisolone (PREDNISOLON ACTAVIS) from 20Nov2020 for Chronic obstructive airways disease, ipratropium bromide, salbutamol sulfate (IPRAMOL) from 20Nov2020 for Chronic obstructive airways disease exacerbated, orphenadrine hydrochloride (LYSANTIN) from 02Dec2019 to 03Jan2021 for Anxiety aggravated, quetiapine fumarate (QUETIAPIN ACCORD) from 16Dec2020 to 03Jan2021 for Psychiatric symptom, salbutamol sulfate (VENTOLINE) from 03Nov2018 for Chronic obstruct airways disease, paracetamol (PARACETAMOL ORIFARM) from 30Nov2020 to 03Jan2021 for Pain, quetiapine fumarate (QUETIAPIN ARROW) from 15Aug2020 to 03Jan2021 for Psychiatric symptom, buprenorphine hydrochloride, naloxone hydrochloride (BUPRENORPHINE/NALOXONE MYLAN) from 29Jun2020 to 03Jan2021 for Opioid abuse, paliperidone palmitate (XEPLION) from 19Dec2019 to 03Jan2021 for Psychiatric disorder prophylaxis, fluticasone furoate, umeclidinium bromide, vilanterol trifenatate (TRELEGY ELLIPTA) from 04Jul2019 to Jul2019 for Chronic obstruct airways disease, promethazine hydrochloride (PHENERGAN) from 24Sep2020 to 03Jan2021 for Insomnia. The patient experienced hypoxic respiratory failure on 31Dec2020, dyspnea exacerbated on 31Dec2020. Patient treatment: On the 31Dec2020 it is recorded that the patient did not want resuscitation in the event of cardiac arrest or respiratory treatment in the event of respiratory failure. Initially the patient did not want to transfer to somatic treatment. But because of anxiety after dyspnoea the patient got treatment with oxygen. On 01Jan2021 the patient denied again treatment despite clear indication for oxygen therapy and COPD exacerbations treatment with ipratropium bromide and salbutamol sulfate (IPRAMOL) and inhalations. On 02Jan2021 the patient received oxygen-treatment, but the patient did not want further somatic treatment. It was stated in the patient journal that the patient did not want treatment and that in the given situation there was nothing more to do. Therefore the patient was returned to department with palliative treatment in the form of oxygen, midazolam subcutaneous (S.C.) and morphine S.C. On the 03Jan2021 the patient’s respiration was calm. The patient was unreachable. At 14:00 he was restless and got palliative treatment with midazolam and morphine. The patient underwent lab tests and procedures which included c-reactive protein: normal on an unspecified date, 16 on 27Dec2020, fibrin D dimer: normal on 31Dec2020, fluid balance assessment: normal on 27Dec2020, forced expiratory volume (FEV 1): 37 % on 2018, hepatic enzyme: normal on 27Dec2020, oxygen saturation: 64 % on an unspecified date, 60 % on 20Dec2020, 58 % on 27Dec2020, 62 % on 31Dec2020, 35 % (in the ambulance) on 31Dec2020, 100 % (on oxygen-treatment) on 31Dec2020, 40-60% on 02Jan2021 12:47 pm, 58 % (in the ambulance) on 02Jan2021 09:00 am, 30 % on 02Jan2021 04:24 am, 99 % (on oxygen-treatment) on 02Jan2021, PCO2 up to 12.8 (Unit not specified) on an unspecified date, PO2 Down to 4.8 (Unit not specified) on an unspecified date. The patient died on 03Jan2021. An autopsy was not performed. The outcome of the events was fatal. Causality: The reporter assessed that even though the patient’s symptoms have occurred long before the vaccination, it can not be ruled out that the patient’s dyspnoea and hypoxia due to COPD have been aggravated by the vaccine. If the Medicines Agency receives supplemental significant information regarding this case the case will be re-submitted.; Reported Cause(s) of Death: Dyspnea exacerbated; Hypoxic respiratory failure | |
| COVID19 VACCINE (COVID19) | Dyspnoea; suspected pulmonary edema; This is a spontaneous report downloaded from the regulatory authority DK-DKMA-WBS-0028304. Report was received from a contactable physician via from the regulatory authority. An 80-year-old female patient received bnt162b2 (COMIRNATY, lot EJ6797, expiration date 30Apr2021), intramuscularly on 03Jan2021 at single dose for covid-19 immunisation. Medical history included dementia with lewy bodies from an unknown date and unknown if ongoing, osteoporosis from an unknown date and unknown if ongoing, hypertension from an unknown date and unknown if ongoing. No previous drug was given. The patient’s concomitant medications were not reported. On 04Jan2021 around 12, approximately 25 hours after the vaccination the patient developed dyspnoea and pulmonary edema. 4 hours later she died. The patient did not experience any allergic symptoms. Events reported as dyspnoea and suspected pulmonary edema. The ADRs were by the reporter reported as fatal. No treatment due to the ADRs was reported. Reported cause of death was pulmonary edema. Outcome of event dyspnoea also reported as not recovered. There was no information regarding test results. It was not reported if an autopsy was performed.; Reported Cause(s) of Death: Pulmonary edema; Dyspnoea | |
| COVID19 VACCINE (COVID19) | Sepsis; Acute bronchopneumonia; This is a spontaneous report received from a contactable physician downloaded from the Regulatory Authority (GB-MHRA-EYC 00236063 and GB-MHRA-ADR 24546059). An 85-year-old female patient received the first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE), intramuscularly, on 15Dec2020 as a single dose for COVID-19 vaccination. The patient’s medical history was not reported. Concomitant medications included pregabalin (MANUFACTURER UNKNOWN), amitriptyline (MANUFACTURER UNKNOWN), amlodipine (MANUFACTURER UNKNOWN), candesartan (MANUFACTURER UNKNOWN), and levothyroxine (MANUFACTURER UNKNOWN). The patient experienced acute bronchopneumonia on 18Dec2020 and sepsis on an unspecified date. The events caused hospitalization and were reported as fatal. The clinical course was reported as follows: The patient was brought to the hospital by ambulance with severe sepsis and bronchopneumonia. She was resuscitated but unfortunately died shortly after arriving. The family reported that the patient received the coronavirus vaccine on 15Dec2020. It was reported that it is unclear from the family history whether she was unwell before she received the vaccine. The clinical outcome of acute bronchopneumonia and sepsis was fatal. The patient died on 19Dec2020. The cause of death was reported as acute bronchopneumonia and sepsis. It was not reported if an autopsy was performed. No follow-up attempts are possible; information on batch number cannot be obtained.; Sender’s Comments: The information available in this report is limited and does not allow a medically meaningful assessment of the case. In particular the following relevant information is not available: medical history, autopsy report.; Reported Cause(s) of Death: Sepsis; Acute bronchopneumonia | |
| COVID19 VACCINE (COVID19) | Lower respiratory tract infection; This is a spontaneous report from a contactable physician downloaded from the Regulatory Authority GB-MHRA-EYC 00236087, Safety Report Unique Identifier: GB-MHRA-ADR 24546153 . A 83-year-old female patient received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE), intramuscularly on 18Dec2020 at single dose for covid-19 immunization. Medical history included vascular dementia from an unknown date and unknown if ongoing, severely frail from an unknown date and unknown if ongoing. This patient was severely frail as a result of vascular dementia and was a permanent nursing home resident. Concomitant medication included amoxicillin, doxycycline, sodium valproate, quetiapine, omeprazole, paracetamol. The patient experienced lower respiratory tract infection (LRTI) on an unspecified date. Patient died on 22Dec2020 within 5 days of receiving Covid vaccine, had been on antibiotics for LRTI for 2 days and had appeared to be improving, temperature was settled before vaccine was administered. She had a negative Covid swab at the onset of her symptoms. It would seem more likely that this patient died as a result of an evolving LRTI than as a result of receiving Covid vaccination. She was changed to amoxicillin 2 days before she died. The other outcome for Death was: Died 22Dec2020 but cause of death felt to be due to LRTI not vaccine. It was not reported if an autopsy was performed. No follow-up attempts are possible, information on batch number cannot be obtained.; Sender’s Comments: The underlying predisposing condition (severely frail, lower respiratory tract infection) have been assessed to have played a major role toward the event.; Reported Cause(s) of Death: Lower respiratory tract infection | |
| COVID19 VACCINE (COVID19) | Death; This is a spontaneous report from a contactable consumer and a physician downloaded from the Regulatory Authority number GB-MHRA-WEBCOVID-20201222043330 and Safety Report Unique Identifier GB-MHRA-ADR 24545938. A 78-year-old male patient received first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE, Solution for injection, LOT: EJ0553) via an unspecified route of administration on 20Dec2020 around 15:45 at single dose in left upper arm for COVID-19 vaccination. The patient ongoing medical history included Depression, Hypertension, chronic obstructive pulmonary disease and ischaemic heart disease. Patient is not enrolled in clinical trial. Patient has not been tested/or has had an inconclusive test for COVID-19. Patient has not had symptoms associated with COVID-19. Concomitant medication included citalopram taken for Depression. The patient was taking unspecified concomitant medications for hypertension, chronic obstructive pulmonary disease (COPD) and ischaemic heart disease. The patient experienced death in Dec2020 (reported as in the evening of the 20Dec2020 or morning of 21Dec2020). Specifically, it was reported that the patient had the first dose of the vaccine at around 15:45 on 20Dec2020 and was observed for 15 minutes after with no side effects, the patient then left the site with family member. He was well that evening, he lived alone but spoke on the phone in the evening and felt well. On the 21Dec2020, after went to check on him and he was found in his bed passed away. When seeing the body, it was assumed that he had passed away in the evening of the 20Dec2020 or morning of 21Dec2020. Although unlikely, it was less than 24 hours after taking the vaccine. Patient has not tested positive for COVID-19 since having the vaccine. The patient was found dead in his flat the next day on 21Dec2020 by next of kin. He was dropped of home by family after the vaccination, he spoke to his family on the night after having the vaccination and told them he was feeling fine and was going to bed. He did not respond to telephone calls the next day (on Monday 21Dec2020) so the family went over to his flat and found he had passed away. The patient was registered at another surgery. Screening questions were asked, no contra indication found. It was not reported if an autopsy was performed. No follow-up attempts are possible. No further information is expected.; Reported Cause(s) of Death: death | |
| COVID19 VACCINE (COVID19) | Patient had a rash prior to COVID vaccine. Prednisone 10mg started on 1/5/21 (only one dose administered) 1/6/21, 1am – Rash worsened with increase redness, warmth and extending of body surface, Temp 100.4, 1/6/21, 1:20am Benadryl 25mg administered, (Keflex was ordered at this time, however never administered). 1/6/21, 3am rash improved, temp 99.1 1/6/21, 6:50am Right facial droop and right sided weakness, sent to ER 1/6/21 transferred to hospital, continues to be hospitalized 1/11/2021 | |
| COVID19 VACCINE (COVID19) | Fever; This is a spontaneous report from a newsletter, from a contactable consumer (profession unspecified). Regulatory authority report number was not provided. An elderly female patient received bnt162b2 (COMIRNATY, Solution for injection, lot number and expiration date not provided), via an unspecified route of administration on an unspecified date at single dose for COVID-19 immunization. Medical history included ongoing dementia in a palliative state. The patient’s concomitant medications were not reported. The verbatim narrative was reported as follows: ‘Status report on suspected side effects from vaccination against covid-19. The report on the second death was received on 05Jan2020. It concerns an elderly female with dementia in a palliative state. The female was vaccinated with Comirnaty, had fever on an unspecified date and passed away three days later. The information in the report is very brief and will seek additional information from the reporter. Currently, has no information on the female’s confirmed cause of death and there is no established causality with the vaccine.’ The patient died on an unspecified date. It was not reported if an autopsy was performed. The outcome of the event was fatal. No follow-up attempts are possible; information about LOT/batch number cannot be obtained.; Reported Cause(s) of Death: had fever and passed away three days later | |
| COVID19 VACCINE (COVID19) | heart attack; This is a spontaneous report from a non-contactable consumer (discovered on news page and heard in news). An elderly female patient (elderly than 90-year old from home for elderly) received bnt162b2 (COMIRNATY) via unspecified route of administration on unspecified date at single dose for COVID-19 immunization (other details not reported). Medical history included heart attack. Concomitant medications were not reported. patient experienced heart attack six hours after vaccination that obviously occurred after repeated heart attack. It was stated that heart attack was not connected to the vaccination. There was no acute allergic reaction. Case was further investigated (independent committee) and confirmed the vaccination was not reason of death. Patient died from heart attack, it was unknown if autopsy was done. Information on batch number has been request.; Sender’s Comments: Fatal heart attack is not related to bnt162b2 use; the advanced old patient had pre-existing medical condition including previous episode of heart attack thus the underlying cardiovascular provided an explanation for the event onset.; Reported Cause(s) of Death: heart attack | |
| COVID19 VACCINE (COVID19) | HPI narrative: Patient was fine until 2 days ago. Patient does have chronic dementia but got Covid vaccine 11:00 2 days ago and that night seem to be confused and not able to walk since then. Patient poor appetite since yesterday. Patient had diarrhea 3 x 2 days ago. No vomiting no fever no cough does not appear to be short of breath. Patient also with left hip pain for few days with no injury.All history obtained from caretaker at bedside. | |
| COVID19 VACCINE (COVID19) | SEVERE ABDOMINAL PAIN | |
| COVID19 VACCINE (COVID19) | “experienced symptoms of TIA; Severe aphasia; blurred vision; confusion; short term memory loss; elevated blood pressure; This is a spontaneous report from a contactable Other-HCP(Patient). A 57-year-old female patient received first dose of BNT162B2(PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration at right arm, on 22Dec2020 08:15 at single dose for COVID-19 immunization. The patient was not pregnant. Medical history included herpes simplex on lips, post menopause, elevated cholesterol w/lifestyle changes. Known allergies reported as no. Concomitant medication included varicella zoster vaccine rge (cho) (SHINGRIX) for immunization. On 04Jan2021 08:30, the patient experienced symptoms of transient ischaemic attack(TIA), severe aphasia, blurred vision, confusion, short term memory loss, elevated blood pressure. The patient admitted to (Hospital name) (still here, hospitalization days reported as 2). Symptoms resolved except very mild aphasia. The patient had very few risk factors for TIA but did have family history of cardiovascular(CV) disease at young age, low density lipoprotein(LDL) was 192. The patient did not have diabetes, HTN, or known heart disease. She did not have severe anxiety. She did not smoke or use any substances. She walked about five miles 4x a week. Weigh reported as 157. Events reported as serious due to hospitalization. The patient had no Covid prior vaccination. Covid(nasal swab) was tested post vaccination on 04Jan2021, Covid test result was Negative. The event resulted in emergency room/department or urgent care. Treatment received for the adverse event included clopidogrel bisulfate(PLAVIX), acetylsalicylic acid (ASPIRIN), statin; potassium, CT, MRI, “”telemet””. The outcome of the events was recovering. Information on the lot/batch number has been requested.; Sender’s Comments: The Company cannot completely exclude the possible causality between the reported symptoms of transient ischaemic attack(TIA), severe aphasia, blurred vision, confusion, short term memory loss, elevated blood pressure and the administration of BNT162B2, based on the reasonable temporal association. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to RA, Agency, as appropriate.” | |
| COVID19 VACCINE (COVID19) | DVT; have pain in same site where DVT is; This is a spontaneous report from a contactable consumer. A 28-years-old female patient received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE, lot number EK9291), via an unspecified route of administration in left deltoid on 24Dec2020 10:00 at first single dose for COVID-19 immunization. Medical history was none. There were no concomitant medications. Caller was calling to report a possible adverse reaction to the Pfizer Covid-19 vaccine. The patient was currently at hospital, she was admitted for deep vein thrombosis (DVT) of left iliac vein, the patient had no past history as to why this would happen, that she is only 28 years old. Received the vaccine on 24Dec2020, the following day she did have pain in same site where DVT was. Took ibuprofen for the pain. The patient was admitted yesterday 04Jan2020 for the DVT, they were currently treating her with Lovenox injections and prescribing dose for discharge is Eliquis. CT scans and three shots of Lovenox for it, doing a doppler of bilateral legs and echocardiogram (echo) of her heart to make sure there is nothing else. The AEs require a visit to emergency room. The patient was asking if she can still get the 2nd dose based off the adverse event she experienced. Outcome of DVT was not recovered, of pain was unknown. | |
| COVID19 VACCINE (COVID19) | Followed by neurological symptoms staring day 4; parasthesias of both upper extremity; progression to muscle weakness of all four extremities/Currently with significant muscle weakness, Left hand weakness leading to dropping of objects and left foot drop; progression to muscle weakness of all four extremities/Currently with significant muscle weakness, Left hand weakness leading to dropping of objects and left foot drop; Flu like symptoms first 3 days; This is a spontaneous report from a contactable physician (patient). A 39-year-old male patient received first dose of bnt162b2 (PFIZER-BIONTECH COVID-19 VACCINE, solution for injection, lot number: ek5730) at left arm, via an unspecified route of administration on 16Dec2020 at single dose for covid-19 immunisation. Medical history included hypertension, diabetes, migraines, Eosinophilic granulomatosis with polyangiitis (EGPA) remission. Prior to vaccination, the patient was not diagnosed with COVID-19. The patient did not have any allergies to medications, food, or other products. The patient’s concomitant medications were not reported. On 21Dec2020, the patient experienced flu like symptoms first 3 days. Followed by neurological symptoms staring day 4, parasthesias of both upper extremity with progression to muscle weakness of all four extremities. Leading to 2 ER visits and hospital admission. Evaluation by internal medicine, neurology and rheumatology. Currently with significant muscle weakness, Left hand weakness leading to dropping of objects and left foot drop. The events resulted in Doctor or other healthcare professional office/clinic visit, Emergency room/department or urgent care, Hospitalization, Disability or permanent damage. The treatment for events included High dose steroid. Covid test included Nasal Swab: negative on 19Dec2020. The outcome of events was not recovered.; Sender’s Comments: Based on temporal association, the causal relationship between bnt162b2 and the events influenza like illness, neurological symptom, paraesthesia, muscular weakness and peroneal nerve palsy cannot be excluded. The information available in this report is limited. This case will be reassessed once additional information becomes available. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees, and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | blasting headaches; chills all night; dry heaving all night; Nausea; no fever but her skin felt hot; sore left arm; This is a spontaneous report from a contactable nurse for herself. This 64-year-old female patient started to receive BNT162B2 (PFIZER-BIONTECH COVID-19 MRNA VACCINE), intramuscular on 18Dec2020 at single dose on left upper arm (lot: EKS730), via an unspecified route of administration on 05Jan2021 14:30 at single dose (lot: EL1284 or EKI284) for COVID-19 immunisation. Medical history and concomitant medications were none. The patient did not have anything with the first shot except a sore left arm on 18Dec2020. She stated that if she lays on left side it is sore. The patient had the sore left arm both times that she got the vaccine. She got second dose of vaccine on 05Jan2021 at 2:30pm and had a blasting headache and just had chills that went away about hour ago on 05Jan2021. She did not have a fever but her skin felt hot on 05Jan2021. She stated that she had dry heaves on 05Jan2021. The patient started that she had a blasting headache within a few hours of the vaccine and it gradually got worst by the time she went to bed. Stated that the chills and dry heaves started then and throughout the night. The chills stopped an hour before she got up. Stated that she went to check her temperature and did not have a fever despite having chills and her skin feeling hot. Stated that nausea started about 10 at night on 05Jan2021. Seriousness for blasting headache, chills and dry heaves was disabling, for nausea was medically significant, for other events was non-serious. The patient took Ibuprofen for the headache. Stated that she was going to try to drink something. The outcome of sore left arm was not recovered; of chills was recovered on 06Jan2021. The outcome of other events was recovering. The causality for blasting headache, chills, dry heaves, nausea and skin felt hot was related (Source of assessment: Primary Source Reporter, Method of assessment: Agency Information on the batch number has been requested.; Sender’s Comments: Based on available information, a possible contributory role of the subject product, BNT162B2 vaccine, cannot be excluded for the headache, chills, dry heaves and other reported events due to temporal relationship. There is limited information provided in this report. Additional information is needed to better assess the case, including complete medical history, diagnostics, counteractive treatment measures and concomitant medications. This case will be reassessed once additional information is available. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | Joint pain /felt like it was worsening joint pain; just severe pain to where she couldn’t walk; This is a spontaneous report from a contactable Other HCP. A 70-year-old female patient received BNT162B2(Lot Number: ET1685), via an unspecified route of administration at Deltoid Left on 23Dec2020 08:00 at the 70 years old at single dose for COVID-19 immunization. The medical history included rheumatoid arthritis. The concomitant medications were none. The patient received the shot on 23Dec2020 and experienced Joint pain afterward on 02Jan2021. The patient did have rheumatoid arthritis so there was that. The patient felt like it was worsening joint pain on 02Jan2021. She has had no fever, just severe pain to where she couldn’t walk on 02Jan2021. The joint pain has gotten worse and it has gotten to where she is going to advise her not to take the second shot. The Reporter assessed the seriousness for the events was Disabling. The events did not require a visit to Emergency Room but required a Physician Office visit on 06Jan2021. The patient received a steroid injection on 06Jan2021. There was none History of all previous immunization with the Pfizer vaccine considered as suspect. There was none Additional Vaccines Administered on Same Date of the Pfizer Suspect. There was no Prior Vaccinations within 4 weeks. The patient underwent lab tests and procedures, which included x-rays on 06Jan2021: unknown results (they were awaiting the X-rays). The outcome of the events was not recovered. The information on the batch number has been requested.; Sender’s Comments: Based on the available information the events worsening joint pain and walking difficulty are attributed to underlying Rheumatoid arthritis; however, based on a compatible temporal association, contributory role of BNT162B2 vaccine to events occurrence cannot be completely excluded. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | Acute demyelinating encephalomyelitis; Slurring his speech; Stroke; This is a spontaneous report from a contactable physician. A 35-year-old male patient received first dose bnt162b2 (PFIZER-BIONTECH COVID-19 VACCINE), intramuscular in deltoid (unknown if right or left) on 17Dec2020 at 30 ug, single for ‘Preventative’. Medical history included hypertension. There were no concomitant medications.(Physician) He is calling about the Pfizer Covid 19 vaccine. States what is going on with the patient may be associated as a side effect. The patient got the vaccine two to three weeks ago, he clarifies the patient received the vaccine on 17Dec2020 and the patient ended up acutely developing (states it is a presumptive diagnosis) Acute demyelinating encephalomyelitis, states it looks like a radiologic diagnosis. The patient is an employee at hospital. When querying seriousness states it is medically significant but could be disabling but he thinks the patient will recover. Reporter seriousness for acute demyelinating encephalomyelitis: Medically significant, Hospitalization. Patient was hospitalized on Sunday and he is still admitted at this time. Dates when patient was in hospital for acute demyelinating encephalomyelitis was from 03Jan2021 to ongoing. Caller thinks the patient was flown to (Place) yesterday. The patient’s mother asked the caller if the caller thought the acute demyelinating encephalomyelitis was from the vaccine and the caller responded that he did not think it was from the vaccine. He confirms the patient is still admitted in the hospital and the patient’s attending neurologist is doctor. The caller heard about the patient from doctor. When querying covid vaccine dose, the caller states the standard dose is 30 mcg. This was clarified and documented as provided. The patient has not received his second dose yet. He asks if the patient should receive the second dose. He asks a general question if a pregnant patient can be given the Pfizer covid vaccine. He heard the patient had a stroke then the CFO tried to talk to him and the patient was slurring his speech. Caller spoke to the patient’s mother this morning and caller told the mother that he would try to find out what is going on with the patient. He asked that the patient get an HIV test even though he does not think the patient is at risk. Vaccination facility type was Hospital. Vaccine administered at military facility was No. None additional vaccines administered on same date of the PFIZER suspect. AE acute demyelinating encephalomyelitis require a visit to Emergency Room, not visit to physician office. Prior Vaccinations (within 4 weeks) was none. He has heard of acute demyelinating encephalomyelitis being associated with vaccines in the past and states that it is rare and usually in kids. States he saw patients that may have had acute demyelinating encephalomyelitis back in the 80s and 90s. Therapeutic measures were taken as a result of acute demyelinating encephalomyelitis (Patient will get steroids tonight pending the review of the x-ray). The outcome of the events was unknown. Information on the lot/batch number has been requested.; Sender’s Comments: The reported stroke with speech slurred, and the presumptive diagnosis of acute demyelinating encephalomyelitis (looks like a radiologic diagnosis by the reporting physician), was most likely an intercurrent disease, and unlikely causally related to the first dose bnt162b2 (PFIZER-BIONTECH COVID-19 VACCINE). The case will be reassessed should additional information become available. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | “Swollen lymph nodes; blood pressure was 90/50; Chills; body aches; Fatigue; Joint pain; Bone pain; Headache; Swelling, pain, redness and soreness at the injection site; Swelling, pain, redness and soreness at the injection site; Swelling, pain, redness and soreness at the injection site; Tachycardia / heart rate was 144-152; painful to breath and she was grunting; also said that the injection site itched a little bit.; Fever; First dose on 17Dec2020, second does on 04Jan2021; This is a spontaneous report from a contactable nurse reporting for herself. A 35-year-old female patient received the first single dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE) ( (lot number was unknown) intramuscularly on 17Dec2020 at 0.3ml, and the second single dose (lot number EJ1685) intramuscularly on 04Jan2021 07:45 at 0.3ml, for COVID-19 prophylaxis. Medical history included ongoing diabetes. Her diabetes was diagnosed about 6 months ago (reported on 06Jan2021) and was well controlled. Her glucose was at 140 at the ER (emergency room) even after drinking a drink that had sugar in it. The patient’s concomitant medications were not reported. The patient received the second dose on 04Jan2021 at 07:45 and experienced a severe reaction hours after she received it, and she ended up having to go the ER and that they were baffled about what happened. She reported her symptoms were: fatigue, joint pain mainly in her knees, bone pain that did not feel like muscle pain, headache, swollen lymph nodes in the left axilla, and swelling, redness, pain, and soreness in the left arm at the injection site and also said that the injection site itched a little bit. Her symptoms started about 9-10 at night on 04Jan2021. She said that she felt like with the second dose she noticed the soreness and pain and redness at injection site after about 2 hours after receiving the second dose, which was sooner than with the first injection. The fever started with the chills and body aches. The chills and body aches started at 22:00 on 04Jan2021. She took acetaminophen and melatonin to try and sleep it off. Fever was after that at around midnight 04Jan2021 and she was burning in fever and her temperature was 104. She stated she started taking layers off and got out from under her covers. She had a sweater on when she took her temperature and her axillary temperature was 105. She said that she got into a hot shower because that was the only thing that provided comfort to her chills. She had not had any chills in about the last 12 hours though (as reported on 06Jan2021). She said that the body aches were so severe that she just sat in her bed and cried. She had tachycardia and she was grunting in pain. She was rotating ibuprofen and acetaminophen and then her fever was not as high. She stated that if her fever came back it was lower each time, and if she did not take the medications though, the bone pain was excruciating. She said that with the bone pain it was like no-one can hold her hand or hug her. Fatigue started at around midnight 04Jan2021. Her headache was at midnight. She felt like she had a headband on and was radiating down the back of her neck. She noticed the lymph nodes were swollen after she went to the ER at around 07:00-08:00 05Jan2021. She was walking with pillow under her arm, and if she moved or lifted her arm it hurt. She went to the ER at 01:00 05Jan2021. Her blood pressure was 90/50, her heart rate was 144-152 at rest, temperature was 102.8, O2 saturation was 95-96. She said that it was painful to breath and she was grunting, but did not have any breathing issues. The nurse thought she was septic and notified the doctor. The nurse and the doctor did not think it was the vaccine and thought that she was COVID-19 positive. But after they tested her, she was negative for flu and COVID and they were baffled. They were unclear on what happened and did not think it was from the vaccine. They bolused her 1 liter of Normal Saline and gave her a dose of Fentanyl that minimally helped with the bone pain. She was also given 30mg of ketorolac (TORADOL) IV (intravenous) and that significantly improved her pain. She was there a total of 3 hours. She was admitted to the back area of the ER. She said that they drew labs and everything was within normal limits. Her CRP (C-reactive protein) was 30 and her lactate was at 1.5. She said that they told her that she was most likely having an immune response to the vaccine. Her heart rate and blood pressure came to a more normal range and everything returned to baseline. Her heart rate was 109. She was told to rotate paracetamol (TYLENOL) and ibuprofen for at least the next 24 hours and hydrate. The events fever, chills, fatigue, joint pain, bone pain, headache, swollen lymph nodes, and swelling, pain, redness and soreness at the injection site were serious due to hospitalization from 05Jan2021 to 05Jan2021. The patient was recovering from fever, chills, joint pain, headache, heart rate and blood pressure, not recovered from fatigue, bone pain, swollen lymph nodes and swelling, pain, redness and soreness at the injection site. The event swollen lymph nodes worsened. The outcome of “”injection site itched a little bit”” was unknown. The reporter considered the events fever, chills, fatigue, joint pain, bone pain, headache, swollen lymph nodes, and swelling, pain, redness and soreness at the injection site were all related to the vaccine; Sender’s Comments: Based on temporal association, the causal relationship between BNT162B2 and the events pyrexia, chills, fatigue, pain, arthralgia, bone pain, headache, lymphadenopathy, vaccination site erythema, vaccination site swelling, vaccination site pain, blood pressure decreased, tachycardia, grunting and vaccination site pruritus cannot be excluded. The information available in this report is limited and does not allow a medically meaningful assessment. This case will be reassessed once additional information becomes available. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees, and Investigators, as appropriate.” | |
| COVID19 VACCINE (COVID19) | Death; This is a spontaneous report from a contactable Physician. An elderly male patient received BNT162B2 (COVID vaccine), via an unspecified route of administration on an unspecified date in Dec2020 at single dose for COVID-19 immunisation. The patient’s medical history and concomitant medications were not reported. The patient experienced death in Jan2021. It was unknown if an autopsy was performed. It was unknown if any treatment was received for the event. It was unknown if the patient was diagnosed with COVID prior vaccination or if the patient had been tested for COVID post vaccination. Seriousness criteria for the event was reported as death and hospitalization. Pfizer is a marketing authorization holder of [COVID vaccine] in the country of incidence or the country where the product was purchased (if different). This may be a duplicate report if another marketing authorization holder of [COVID vaccine] has submitted the same report to the regulatory authorities. Information about lot/batch number has been requested.; Sender’s Comments: Current information is very limited for full assessment. Further information such medical history, concomitant medications, concurrent illness and event term details especially death cause and autopsy results are needed for meaningful evaluation. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate.; Reported Cause(s) of Death: Death | |
| COVID19 VACCINE (COVID19) | COVID-19; COVID-19; Pneumonia; respiratory failure; This is a spontaneous report from a contactable consumer. An 80-year-old female patient received first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE) via an unspecified route of administration on 02Jan2021 for COVID-19 immunization. Medical history included Alzheimer’s and others. No known allergies. Concomitant medications included unspecified medications. The reporter’s mother in law was tested for COVID-19 at a nursing facility on 25Dec2020 and she was negative. On 02Jan2021, she received the first dose of Pfizer vaccine. On 04Jan2020, she developed a high fever, needed oxygen and was positive for COVID-19. Date of death was 04Jan2021. The cause of her death was listed as pneumonia, respiratory failure and COVID-19. No autopsy performed. No treatment received. No one knew if the vaccination contributed to her death. It was hard to know if her death was due to the administration of the vaccine or it exacerbated the COVID19 symptoms which led to her death. Since this was unknown, it could have been a possibility. The reporter wanted to give us this information because we might want to consider having high risk population, patients with underlying conditions, older population tested for COVID-19 prior to the vaccination, as this is not currently a recommendation or a requirement. All is very new and they are all learning so the reporter wanted to share this information with us. The patient did not receive any other vaccines within 4 weeks prior to the COVID vaccine. There are medications the patient received within 2 weeks of vaccination. Prior to vaccination, the patient was not diagnosed with COVID-19. Since the vaccination, the patient has been tested for COVID-19. The outcome of the events was fatal. Information about Lot/Batch has been requested.; Sender’s Comments: The association between the fatal event lack of effect (pneumonia, respiratory failure and COVID-19) with BNT162b2 can not be fully excluded. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to regulatory authorities, Ethics Committees, and Investigators, as appropriate.; Reported Cause(s) of Death: Pneumonia, respiratory failure and COVID-19; Pneumonia, respiratory failure and COVID-19; Pneumonia, respiratory failure and COVID-19; Pneumonia, respiratory failure and COVID-19 | |
| COVID19 VACCINE (COVID19) | he passed away; not responsive; mind just seemed like it was racing; body was hyper dried; Restless; not feeling well; ate a bit but not much; kind of pale; Agitated; Vomiting; trouble in breathing; This is a spontaneous report from a contactable consumer (brother of the patient). A 54-year-old male patient received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration, on 04Jan2021 (at the age of 54-years-old) as a single dose for COVID-19 immunization. Medical history included diabetes and high blood pressure. Concomitant medications included metformin (MANUFACTURER UNKNOWN) taken for diabetes, glimepiride (MANUFACTURER UNKNOWN) taken for diabetes, lisinopril (MANUFACTURER UNKNOWN), and amlodipine (MANUFACTURER UNKNOWN). The patient experienced not feeling well, ate a bit but not much, kind of pale, vomiting, trouble in breathing, and agitated on 04Jan2021; body was hyper dried and restless on 05Jan2021; mind just seemed like it was racing on 06Jan2021; and not responsive and he passed away on 06Jan2021 at 10:15 (reported as: around 10:15 AM). The clinical course was reported as follows: The patient received the vaccine on 04Jan2021, after which he started not feeling well. He went right home and went to bed. He woke up and ate a bit but not much and then was kind of pale. The patient then started to vomit, which continued throughout the night. He was having trouble in breathing. Emergency services were called, and they took his vitals and said that everything was okay, but he was very agitated; reported as not like this prior to the vaccine. The patient was taken to urgent care where they gave him an unspecified steroid shot and unspecified medication for vomiting. The patient was told he was probably having a reaction to the vaccine, but he was just dried up. The patient continued to vomit throughout the day and then he was very agitated again and would fall asleep for may be 15-20 minutes. When the patient woke up, he was very restless (reported as: his body was just amped up and could not calm down). The patient calmed down just a little bit in the evening. When the patient was awoken at 6:00 AM in the morning, he was still agitated. The patient stated that he couldn’t breathe, and his mind was racing. The patient’s other brother went to him and he was not responsive, and he passed away on 06Jan2021 around 10:15 AM. It was reported that none of the symptoms occurred until the patient received the vaccine. Therapeutic measures were taken as a result of vomiting as aforementioned. The clinical outcome of all of the events was unknown; not responsive was not recovered, the patient died on 06Jan2021. The cause of death was unknown (reported as: not known by reporter). An autopsy was not performed. The batch/lot number for the vaccine, BNT162B2, was not provided and has been requested during follow up.; Reported Cause(s) of Death: not responsive and he passed away | |
| COVID19 VACCINE (COVID19) | 26-year-old lady came in after she noticed she had bruises on her left hand after a CPR procedure at hospital. Patient was apparently in well health, she had received COVID-19 mRNA vaccine on January 7 at 3 PM, she has taken 2 pills with ibuprofen and tylenol for pain in right deltoid following vaccination. She was doing the CPR at 1:00 this afternoon, and she noticed that her left dorsum had some bruises. She took day off and went home and noticed that she also had bruises in both medial thighs, above the knee and some bruises in scalp. Patient presented to the Emergency Room 1/9/2021 ~6PM and platelet count was found to be 2×10^3/uL. Patient required transfusion of 7 units of platelets, steroids, and IVIG. | |
| COVID19 VACCINE (COVID19) | SOB, Sleeplessness, | |
| COVID19 VACCINE (COVID19) | Scratchy throat, dizziness and eventually feeling like her throat is closing in | |
| COVID19 VACCINE (COVID19) | Patient was reported to be deceased at home by law enforcement on 1/7/21 | |
| COVID19 VACCINE (COVID19) | “Patient presents with abdominal pain that started in the middle of the night. Had first COVID vaccine the previous day. Patient states the pain is intermittent “”comes and goes”” “”cramping”” “”pressure and bloating”” feeling. Patient states her normal bowel movements are 12 times per day. The last time she went was this morning. She is concerned about an “”obstruction”” Patient states she has “”some nausea”” She states she has ate and drank normally today. Patient has a history of ulcerative colitis and a complete colectomy with a ileal rectal pouch. She has had abdominal pain since this morning which is crampy, associated with nausea and recurrent vomiting. She normally has 6-12 bowel movements a day, but none since this morning. She does feel her abdomen is distended as well. The last time she had anything like this was when she developed pouch itis last spring, but that was much less painful than this. Her appendix is gone, but she believes she still has her gallbladder.” | |
| COVID19 VACCINE (COVID19) | Went into the ED for bilateral hand and feet tingling. Worked up for possible Guillain Barre. | |
| COVID19 VACCINE (COVID19) | “Patient reported stroke-like symptoms as he was driving to work: started “”feeling funny”” with dizziness; progressed to feeling weakness in left side of face with facial drooping; tingling in left hand progressed to numbness and weakness in left arm and leg; difficultly coordinating motor movement in right arm; difficulty/ diminished speech “”tongue felt fat.”” Symptoms resolved within appx 1+ hour from onset. Per patient, he was given an injection at the hospital (unsure for what?) and was discharged with a prescription for chlorthalidone 25mg daily, blood pressure was elevated.” | |
| COVID19 VACCINE (COVID19) | There were no adverse reactions. Resident Died, she had a history of issues with her health prior to the vaccine. | |
| COVID19 VACCINE (COVID19) | Patient was found unresponsive at home with SpO2 20% 1/2/2021 | |
| COVID19 VACCINE (COVID19) | 1/6/21 8pm started with Nasuea, vomiting, diarrhea and fever. 1/7/21 started having intermittent chest pain in the morning. Then in the evening it became constant. Went to ER that evening due to chest pain. EKG showed t wave abnormality. 1st Trop was negative went from 0.08 to 2.3 Had 2 Echo’s done and they were normal. Platelets were 85. Was discharged without chest pain. Troponin on discharge was 0.67 and platelets 61. Was admitted due to Chest pain and troponin. Attending provider diagnosed as myocarditis and thrombocytopenia R/T vaccine. | |
| COVID19 VACCINE (COVID19) | right after vaccine was given i got a head to toe hot flush. i thought it was just anxiety. within 2 minutes i had expolsive diarrhea, felt dizzy. looked in the mirror and saw my neck and chest covered in red rash and hives. felt hot flush again. dr came in noticed hives all over both my arms as well. felt sob and if someone was holding my neck with their hand. given benadryl and epi taken to local er. | |
| COVID19 VACCINE (COVID19) | Patient received the 1st dose of Moderna and was found deceased in her home the next day. | |
| COVID19 VACCINE (COVID19) | Pfizer COVID-19 Vaccine A couple hours after the vaccination the patient experienced pain in the vaccine arm, headache, and feeling ache. Day 1 post vaccination patient experienced sore arm, headache, low grade fever, feeling ache, and GI symptoms with diarrhea. Day 2 post vaccination patient experienced sore arm, Migraine, and diarrhea. Day 3 post vaccine patient woke up with chest pain that radiated into her left arm and some weakness. Patient’s blood sugar was >500 and was admitted to hospital for DKA. | |
| COVID19 VACCINE (COVID19) | My mother was given Pfizer vaccine on Thursday and she died 3 days later yesterday on Sunday!!! | |
| COVID19 VACCINE (COVID19) | Extremely tired went to bed at 8:30. At 11:00, tossed and turned until 12:16 a.m. Did not think she could stand up. Walked in the bathroom had pain in stomach, was very hot and freezing at the same time, was shaking. Head hurt was hurting so bad. Tried standing up from toilet, was passed out. Eye, half of face is bruised and swollen , eye shut for two days. More than forty minutes passed before she could call her husband. Does not remember anything because she was passed out. Husband came in and grabbed her, to get her back into bed. she could not stand. Went to ER at Hospital. Had CT scan, blood work , EKG, chest xray. Was still kind of out of it but was able to communicate with nurses. Face is still black and blue. Last night got up at 4:00 a.m. something was wrong with her throat. Felt like a walnut was in her throat. Went to doctor this morning. They said it was a reaction from the shot. Got an EpiPen, prednisone. Call them in 2 days to let them know how she’s doing and also wear a heart monitor because they do not know how to take care of her, Has had vision blurriness in both eyes. Has not felt well at all. | |
| COVID19 VACCINE (COVID19) | Difficulty breathing, death. | |
| COVID19 VACCINE (COVID19) | Noted left lymph nodes left neck. On January 7, 10 days post injection had acute appendicitis requiring emergency appendectomy. | |
| COVID19 VACCINE (COVID19) | Metallic taste in the back of throat between 15-20 minutes post vaccination, noticeable swallowing and throat irritation at 20-25 minutes post vaccination, tongue and lip numbness and throat tightness at 25-30 minutes, dry hacking cough at 30 minutes. Treated in the ED approximately 1 hour post vaccination, at time of arrival in respiratory distress with subcostal retractions, coughing, speaking 1-2 word sentences, with tachycardia and tachypnea. Treated with IM epinephrine, IV solumedrol and IV Benadryl and IV Benadryl with marked improvement in symptoms. | |
| COVID19 VACCINE (COVID19) | Woke up 1/4/21 with right sided facial weakness consistent with Bell’s Palsy. Started high dose Prednisone and Valtex. Received IVIG infusion on 1/6/21 and 1/7/21. Mild improvement 1/11/21. | |
| COVID19 VACCINE (COVID19) | About 15 minutes after vaccine, hr 155, fever 102, covered in hives, sick to her stomach and a pounding headache. Has had headache since then and been extremely fatigue. | |
| COVID19 VACCINE (COVID19) | Anaphylaxis within 5 minutes of dose given. Tachycardia 130-140s, hot body temperature, trouble swallowing, lightheaded/dizzy, ekg changes, feeling like I was going to pass out even when in bed. IV fluids, benedryl, soul-medrol, famotadine and IM epi given. | |
| COVID19 VACCINE (COVID19) | Trouble swallowing, tingling around the mouth within 5 minutes of vaccine administration. IV started with 25mg Benadryl within 5 minutes of symptom onset. Transfer to ER at 1430. Symptoms unresolved, hr – 120, bp 140/100, O2 sats 100, resp: 21 Additional 25mg Benadryl, 125mg solumedrol, 1ml Ativan given IV at 1435. Symptoms began to resolve, patient discharged at 1600 to home with instructions to return if needed. Patient returned to ER Sunday January 10 at 1300 complaining of throat tightness. Patient was seen by doctor, no acute distress and airway issues seen. Patient elected to stay for 50mg benadryl and 40mg prednisone PO. Patient was discharged to home with script for 40mg prednisone q day for 3 days. Patient feels any remaining allergic symptoms have resolved. | |
| COVID19 VACCINE (COVID19) | RESIDENT 1ST DOSE OF MODERNA VACCINE ADMINISTERED ON 01/04/2021 AT 8:30PM, RESIDENT FOUND UNRESPONSIVE ON 01/05/2021. | |
| COVID19 VACCINE (COVID19) | Herpatic infection left eye causing a herpatic dendrite | |
| COVID19 VACCINE (COVID19) | One week after administration, I had sudden onset inability to move left arm. I was transported to ER immediately. Treated, scanned with CT of brain, MRI of brain, c-spine and brachioplexus. In hospital for 2 days and no answers. Still no answers to left arm paresthesia and proprioreceptor deficits. Spreading into left leg and mild systemic symptoms. I have been to the ER, seen by primary physician, Physiatrist and Neurology and Occupational Therapy. I am scheduled for many more appointments and trying to find and answer. | |
| COVID19 VACCINE (COVID19) | Myocardial infarct; Circulatory collapse; This is a spontaneous report from a contactable physician from the regulatory authroity. The regulatory authority report number is GB-MHRA-ADR 24553112 and GB-MHRA-WEBCOVID-20210104143047. An 82-year-old male patient received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE; Lot number: EJ1688), via an unspecified route of administration, on 31Dec2020 at a single dose for COVID-19 vaccination. Medical history included mitral valve incompetence from 08May2020, myocardial ischaemia from 07May2020, acute myocardial infarction from 07May2020, cataract from 29Nov2019, chronic kidney disease from 03Oct2013, colitis ischaemic from 23May2013, basal cell carcinoma from 20Apr2012, transurethral bladder resection on 06Sep2005, neoplasm malignant (other/unspecified site) from 16Aug2005, debridement (arthroscopic debridement of patella) on 12Jan2005, and essential hypertension from 2005. The patient had not had symptoms associated with COVID-19. The patient was not been tested/or had an inconclusive test for COVID-19. The patient was not enrolled in clinical trial. Concomitant medications included allopurinol (MANUFACTURER UNKNOWN), atorvastatin (MANUFACTURER UNKNOWN), betamethasone valerate (BETNOVATE), bisoprolol (MANUFACTURER UNKNOWN), furosemide (MANUFACTURER UNKNOWN), glyceryl trinitrate (MANUFACTURER UNKNOWN), loperamide (MANUFACTURER UNKNOWN), omeprazole (MANUFACTURER UNKNOWN), phenoxymethylpenicillin (MANUFACTURER UNKNOWN), and ramipril (MANUFACTURER UNKNOWN). The patient experienced myocardial infarct and circulatory collapse on 31Dec2020. The event, myocardial infarct, was reported as fatal. It was reported that the patient collapsed at home the evening after vaccination. The clinical outcome of myocardial infarct was fatal and of circulatory collapse was not recovered. The patient died on 31Dec2020. The cause of death was reported as myocardial infarct. It was unknown if an autopsy was performed. No follow-up attempts are possible. No further information is expected.; Reported Cause(s) of Death: Myocardial infarct | |
| COVID19 VACCINE (COVID19) | anaphylaxis; throat tightening; throat tightening/tingling; throat tightening/tingling/soreness; dry wheezy cough a little dizziness; dizziness; tachycardia; Itching; chills; numb R foot; Low grade temp; h/a today; This is a spontaneous report from a contactable Nurse (patient). A 51-years-old female patient (no pregnant) started to receive bnt162b2 (PFIZER-BIONTECH COVID-19 VACCINE, lot number el3248), via an unspecified route of administration on 06Jan2021 11:00 at the first single dose at left arm for covid-19 immunisation. Medical history included supraventricular tachycardia, adrenal insufficiency, hypothyroidism, attention deficit hyperactivity disorder, hypermobility syndrome, developmental hip. Concomitant medication included hydrocortisone, trazodone, levothyroxine sodium (LEVOTHROID), bupropion hydrochloride (WELLBUTRIN). The patient previously took erythromycin, morphine and experienced drug hypersensitivity. The patient experienced anaphylaxis, throat tightening/tingling/soreness, dry wheezy cough a little dizziness and tachycardia. Itching, numb R foot, Low grade temp and chills and headache on 06Jan2021 11:15. Seriousness criteria reported as life threatening. Taken to ER had IV benadryl, solumedrol, pepcid for anaphylaxis. Placed on O2 and given albuterol nebulizer. Had IV fluid bolus. Now on benadryl and 5 days of prednisone. The patient felt completely fine prior to vaccine. The patient underwent lab tests and procedures which included sars-cov-2 test: negative on 06Jan2021. The outcome of events was recovering. No other vaccine in four weeks; No covid prior vaccination.; Sender’s Comments: A possible causal association between administration of BNT162B2 and the onset of anaphylaxis presented as throat tightening/tingling/soreness, dry wheezy cough a little dizziness and tachycardia. Itching, numb R foot, Low grade temp and chills and headache cannot be excluded, considering the plausible temporal relationship and the known adverse event profile of the suspect product. The underlying predisposing condition of drug allergies may put the patient at high risk of anaphylactic reactions. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | Soreness at injection site started at 1600 Body aches, headache, and low grade fever woke me up around 0100 | |
| COVID19 VACCINE (COVID19) | Anaphylactic reaction; Flushed; Diaphoretic; redness and rash; hives on chest; Tachycardia; shortness of breath; Chest tightness; Dizziness; Headache; This is a spontaneous report from a contactable nurse, the patient. A 47-year-old female patient received the second dose of BNT162B2 (PFIZER-BIONTECH COVID-19 mRNA VACCINE; Lot Number: EL1283), via an unspecified route of administration on 08Jan2021 at 08:49 (at the age of 47-years-old) as a single dose for COVID-19 immunization. There were no known medical history or concomitant medications. The patient previously received the first dose of BNT162B2 on 18Dec2020 (Lot Number: EK5730) for COVID-19 immunization and experienced nausea, headache, and fatigue. On 08Jan2021, about 5-10 minutes after the second dose, the patient experienced anaphylactic reaction, flushed, diaphoretic, redness and rash, hives on chest, tachycardia, shortness of breath, and chest tightness, reported as life-threatening. She reported that these events occurred within less than 10 minutes of receiving the vaccine. She went to the emergency room and was treated with methylprednisolone (SOLUMEDROL), diphenhydramine hydrochloride (BENADRYL), famotidine (PEPCID), and epinephrine (MANUFACTURER UNKNOWN). She was sent home and prescribed methylprednisolone and epinephrine (EPI-PEN). Later on 08Jan2021, she experienced dizziness and headache, which were consistent. She stated she would most likely take ibuprofen (MOTRIN) as treatment (not specified if taken). The clinical outcomes of the flushed, diaphoretic, redness and rash, hives on chest, tachycardia, shortness of breath, and chest tightness were recovered on 08Jan2021; while the outcomes of the dizziness and headache were not recovered and that of the anaphylaxis was reported as recovering.; Sender’s Comments: The reported information is limited. Based on the close temporal relationship and the description of the events, there is a reasonable possibility that the events are related to BNT162 vaccine. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to regulatory authorities, Ethics Committees, and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | Approx 10-15 post vaccine, employee said she felt lightheaded and like her heart was racing. Within 10 minutes she said she felt difficulty breathing, She then vomited. The observation nurse at the clinic administered Epi Pen and called a Code. The employee was transported to the Emergency Dep’t and then to intensive care. She was placed on an Epi drip. | |
| COVID19 VACCINE (COVID19) | loss of consciousness Narrative: Patient received COVID-19 vaccine dose #1 on 1/6/21 w/o complications. Per 1/6/21- 1/9/21 nursing notes, patient did not experience any injection site reactions, denied pain or tenderness at injection site, no dizziness, no n/v, remained afebrile. Around 1/9/21 @1810, patient became acutely nonresponsive after being helped to the edge of bed. Per nurses, he was previously awake/alert, talking and asymptomatic. Patient is DNR/DNI but facility rapid response emergency team called d/t patient’s sudden change of condition. Emergency team helped patient into lying position. Per 1/9/21 ICU emergency team note, patient appeared comfortable w/ no palpable radial pulse and had minimal shallow agonal breathing. Pulse ox 94%, HR in 60s per machine. BP unmeasurably low by BP cuffx3. Resident passed at 18:20 pm. | |
| COVID19 VACCINE (COVID19) | Approximately 1 – 2 hours after receiving I had numbess and soreness to my neck. A few days later started experiencing tingling, buzzing, weakness and heaviness to my right arm and leg. I reported this to my MS doctor who ordered an MRI of the brain and told me to report to you | |
| COVID19 VACCINE (COVID19) | Patient received the vaccine on 12/22/20 without complication. It was reported today that the patient was found unresponsive and subsequently expired at home on 1/11/21. | |
| COVID19 VACCINE (COVID19) | Increased weakness leading to a fall and fever of 101.3 | |
| COVID19 VACCINE (COVID19) | Patient began experiencing fevers, body aches, back pain, fatigue, chills the night she received the vaccine. Symptoms progressed for the following four days when she was ultimately seen in PCP office. Laboratory evaluation demonstrated atypical pneumonia and elevated WBC count. Patient was diagnosed with Acute Myeloid Leukemia via bone marrow biopsy and is receiving treatment at the hospital. | |
| COVID19 VACCINE (COVID19) | LEFT FACIAL AND TONGUE NUMBNESS – WORK UP CVA NEG Narrative: Developed left facial and tongue numbness 4 hours after vaccine – went to ER and admitted for 2 days, negative workup for CVA or other acute etiology. Symptoms resolved prior to discharge from hospital | |
| COVID19 VACCINE (COVID19) | Reported feeling faint and nauseated during observation period. Placed on cart in trendelenberg | |
| COVID19 VACCINE (COVID19) | Within 24 hrs, developed headaches, burning sensation down spine and neck. fullness in head intensified next day. balance was off, numbness and tingling throughout body. Went to ER. Diagnosed Paresthesia | |
| COVID19 VACCINE (COVID19) | The facility had positive cases of COVID when we were able to begin vaccinating residents. Within about a week of vaccination, patient was tested positive for COVID. He was 91 years old and his immune system did not have the time to allow the vaccine to begin working before exposure. His age was a major contributing factor to his death. | |
| COVID19 VACCINE (COVID19) | The facility had positive cases for COVID 19 when the vaccine was received and administered to patient. With her advanced age and chronic conditions, she did not have time to build immunity between the time of vaccination and her testing positive. | |
| COVID19 VACCINE (COVID19) | The facility had a number of positive COVID 19 cases prior to patients vaccination. Due to her advanced age, chronic condition, and exposure, patient did not have the time to build immunity after exposure before becoming positive. | |
| COVID19 VACCINE (COVID19) | Within approximately 30 minutes after vaccine (Thursday), patient presented with red rash to upper chest, severe headache, dizziness and nausea. Was treated with Benadryl and Tylenol per anesthesia onsite. Symptoms remained throughout the day with minimal improvement. The following morning (Friday) only headache remained. patient began having abdominal pain, and was admitted to hospital the next day (Saturday). It is now Tuesday and patient is still hospitalized. Unclear if this entire event is vaccine related. | |
| COVID19 VACCINE (COVID19) | Pt expired due to possible cardiac arrest. Unsure if this was vaccine related. | |
| COVID19 VACCINE (COVID19) | Resident was found deceased at approximately 6pm in her apartment | |
| COVID19 VACCINE (COVID19) | Pt experienced the following morning: fever (100 F) chills, malaise, nausea, and numbness in hands at 0624 and 0836. Pt went to hospital at 1000, tx with IV fluids. | |
| COVID19 VACCINE (COVID19) | “In the early morning of Monday, January 11th the patient developed a significant headache with neck pain. She also reported parathesia and tingling in bilateral upper extremities with weakness of the right upper extremity. She reported feeling very anxious and “”wound up””. Patient presented to the Emergency Department 3 hours later. CT of the head/brain, EKG, CBC,CMP, Magnesium and Cardiac profile were performed with no significant findings. Ativan 0.5mg was administered orally. Patient was admitted to the facility for observation. Symptoms gradually resolved with no additional treatment.” | |
| COVID19 VACCINE (COVID19) | unsure if related to vaccine, but was notified by her next of kin that she died on 1/4/2021. No reports of side effects or hospitalization were reported to the facility prior to the notification of death. | |
| COVID19 VACCINE (COVID19) | patient reported expired 1/7/2021 | |
| COVID19 VACCINE (COVID19) | On 12/31/2020, at approximately 00:15, pt developed a fever of 102.9 F and tachycardia with rate of 120. He was treated with acetaminophen. Later in the morning, he complained of nausea, generalized muscle aches, intermittent increase in confusion. At approximately 14:00, he had a fall out of bed and at that time noted to be short of breath, tachypneic. He was taken via ambulance to Emergency Department. From there he was transferred to Hospital for admission with acute respiratory distress, suspected sepsis with lactic acid 7.4 and Bilateral Pulmonary Emboli. He was started on heparin and broad spectrum antibiotics and transitioned to ELIQUIS on 1/3/2021. Infectious etiology of sepsis was unclear. He continued broad spectrum antibiotics with clinical improvement. Abdominal CT scan was obtained due to intermittent nausea, vomiting, abdominal pain, loose stools. His heart rhythm flipped to Atrial Fibrillation with RVR on 1/2 and his rate improved with titration of metoprolol. He was also treated with prednisone for suspected underlying undiagnosed COPD. It is noted in his hospital summary that PEs presumed provoked in the setting of his recent COVID 19 infection. He was discharged from the hospital on 1/8/2021 and readmitted to the Veterans Home. He has been stable. | |
| COVID19 VACCINE (COVID19) | Received Moderna vaccine on 1/5. Had a single episode of vomiting approximately 1 hour after vaccine. Hx of Asthma, started to develop SOB approximately 2 days later. She reached out to PCP and used inhaler with little relief. SOB worsened and she was admitted to the hospital on 1/8/21. Eval was negative for COVID (3 tests completed), flu and pneumonia. She has elevated WBCs and was given steroids and supplemental oxygen. She is improving but remains inpatient. | |
| COVID19 VACCINE (COVID19) | Death in connection with the vaccination and/or COVID-19 disease/positivity; Death in connection with the vaccination and/or COVID-19 disease/positivity; This is a spontaneous report from a contactable physician. This physician reported similar events for two patients. This is the first of two reports. A patient of unspecified age and gender received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration, on an unspecified date at single dose for COVID-19 immunization. The patient’s medical history and concomitant medications were not reported. On an unspecified date, there was death in connection with the vaccination and/or COVID-19 disease/positivity. It was reported that: two deceased were autopsied, whose death was in connection with the vaccination or COVID-19 disease/positivity. The clinical outcome of death in connection with the vaccination and/or COVID-19 disease/positivity was fatal. The patient died on an unspecified date. The cause of death was reported as: death in connection with the vaccination and/or COVID-19 disease/positivity. An autopsy was performed, and the results were not reported.; Sender’s Comments: The association between the event lack of effect (death was in connection with the vaccination or COVID-19 disease positivity) with BNT162b2 can not be fully excluded given the limited information. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to regulatory authorities, Ethics Committees, and Investigators, as appropriate.,Linked Report(s) : DE-PFIZER INC-2021006905 same reporter, same product, same event, different patient; Reported Cause(s) of Death: Death in connection with the vaccination and/or COVID-19 disease/positivity; Death in connection with the vaccination and/or COVID-19 disease/positivity | |
| COVID19 VACCINE (COVID19) | Patient was sent to the ED due to significant hematuria. He was afebrile. | |
| COVID19 VACCINE (COVID19) | Patient presented with myalgias, fevers, and chest pain on 1/10/21 and was found to have diffuse ST elevation and elevation troponin. He was evaluated by cardiology and diagnosed with acute myopericarditis. He was treated with NSAIDs and colchicine. He improved with this treatment and was discharged on 1/12/21 with ibuprofen and colchicine and outpatient cardiology follow up. | |
| COVID19 VACCINE (COVID19) | death following BNT 162b2 vaccination; This is a spontaneous report from a contactable consumer. A patient of unspecified age and gender received BNT162B2 (COMIRNATY; PFIZER-BIONTECH COVID-19 mRNA VACCINE), via an unspecified route of administration on an unspecified date as the first single dose for COVID-19 immunization. The patient’s medical history and concomitant medications were not reported. Death following BNT162B2 vaccination was noted on an unspecified date. The patient died on an unspecified date. It was not reported if an autopsy was performed.; Reported Cause(s) of Death: death | |
| COVID19 VACCINE (COVID19) | Acute cardio-respiratory event and died a few hours later; This is a spontaneous report received from a contactable physician by Pfizer from the Regulatory Agency. The regulatory authority report number is GB-MHRA-WEBCOVID-20210107093111. Safety Report Unique Identifier GB-MHRA-ADR 24565959. An 84-years-old female patient received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE) at single dose, on 04Jan2021, for COVID-19 immunisation. Patient was elderly and frail and gradually declining in mobility, communication and memory over the last 12 months. Relevant medical history also included vascular dementia form an unspecified date and unknown if ongoing. Concomitant medications were unknown. Patient was not enrolled in clinical trial. COVID-19 virus test was performed twice on an unspecified date, in Dec2020 and on 18Dec2020 and the results were negative. On 04Jan2021, at 06:00 PM, the patient experienced acute cardio-respiratory event and died a few hours later. It was unknown if autopsy was done. Since the vaccination, the patient has not been tested for COVID-19. Patient did not have symptoms associated with COVID-19. The patient was kept comfortable in the nursing home in these last few hours. There was no way to know whether the vaccine was to blame at all, it was unlikely. No follow-up attempts are possible, information about lot number cannot be obtained.; Reported Cause(s) of Death: Cardio-respiratory failure | |
| COVID19 VACCINE (COVID19) | severe ITP; This is a spontaneous report from a contactable physician. A 22-years-old male patient received his first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration on 17Dec2020 at single dose for COVID-19 immunization. The patient medical history and concomitant medications were not reported. The patient developed severe ITP (Idiopathic thrombocytopenic purpura) on 20Dec2020, 3 days after first dose of the vaccine on 17Dec2020. He was hospitalized for 3 days and now the patient was healthy with a normal platelet count on an unspecified date. The physician wanted to know if this patient can get the second dose. The outcome of the event was recovered on an unspecified date. Information on the lot/batch number has been requested.; Sender’s Comments: A possible contributory effect of suspect BNT162B2 on reported ITP cannot be excluded. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to regulatory authorities, Ethics Committees, and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | she was transferred to the hospital with tachycardia, congestion, shortness of breath, and is now unresponsive; she was transferred to the hospital with tachycardia, congestion, shortness of breath, and is now unresponsive; she was transferred to the hospital with tachycardia, congestion, shortness of breath, and is now unresponsive; she was transferred to the hospital with tachycardia, congestion, shortness of breath, and is now unresponsive; This is a spontaneous report from a contactable nurse. A 95-year-old female patient received the second dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE, solution for injection), Lot# EH9899 Exipration on 06Jan2021 at 15:00 at SINGLE DOSE at deltoid for COVID-19 immunization. The patient received first dose of the same vaccine BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE, solution for injection), Lot# EH9899 Exipration date : 31Mar20221, on 16Dec2020. Medical history included : cardiac failure congestive, hypertension, cardiac murmur .There were no concomitant medications. The patient previously took cymbalta , vasotec and zocor and experienced drug hypersensitivity. The patient didn’t receive any other vaccines within 4 weeks prior to the COVID vaccine.This nurse,worker at a skilled nursing facility, reported that this patient with a history of heart failure, received her second dose of the Pfizer-BioNTech Covid-19 vaccine yesterday, 06Jan2021, at 3pm. At 7am on 07Jan2021 she was transferred to the hospital with tachycardia, congestion, shortness of breath, and is now unresponsive. Patient was stable prior to vaccination, but will now be transferred to hospice care. The nurse added the patient had the second COVID vaccine on 06Jan2021 yesterday and has now been transport to hospital due to a drastic decline after the shot. It was explained that this morning around 7 am she was transferred to the hospital. She was experiencing tachycardia, shortness of breath, and congestion. The events started this morning around 6-6:30am. The patient was admitted to the hospital. The shot was given at the facility. She received it at 3pm on 06Jan2021, First dose was on 16Dec2020.The caller relays she didn’t know how aggressive the hospital will be for the patient. She was a full code when left and now a DNR and is unresponsive. The patient will be going on hospice care. The causality was reported as related. The outcome of the events was not recovered.; Sender’s Comments: The Company cannot completely exclude the possible causality between the reported tachycardia, shortness of breath, congestion, unresponsive, and the administration of the COVID 19 vaccine, BNT162B2, based on the reasonable temporal association. The patient’s pre-existing medical condition of cardiac failure congestive, hypertension, cardiac murmur are confounding factors. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to RA, IEC, as appropriate. | |
| COVID19 VACCINE (COVID19) | patient developed acute onset of right-sided facial palsy and pain; patient developed acute onset of right-sided facial palsy and pain; Brain MRI done showing T2 hyperintensity in the brainstem and basal ganglia, suspicious for inflammation; This is a spontaneous report from a contactable physician. A 46-year-old male patient received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE, solution for injection), on 18Dec2020 at SINGLE DOSE for COVID-19 immunization. Medical history included psoriasis. No COVID prior vaccination. It was unknown if the patient received any other vaccines within 4 weeks prior to the COVID vaccine.No known allergies. Concomitant medications (received within 2 weeks of vaccination) included fluoxetine. days following vaccination,on 23Dec2020,the patient developed acute onset of right-sided facial palsy and pain. Brain MRI done showing T2 hyperintensity in the brainstem and basal ganglia, suspicious for inflammation. Work-up is ongoing. AE Resulted in: Doctor or other healthcare professional office/clinic visit, Emergency room/department or urgent care, Disability or permanent damage].It was unknown if the event was treated. The event was assessed as serious for Disabling/Incapacitating. The patient had been tested for COVID post vaccination (covid test result-Negative). The outcome of the events werenot recovered Information about lot/batch number has been requested.; Sender’s Comments: A possible causal relationship between acute onset of right-sided facial palsy and pain with MRI findings suspicious for brain inflammation and BNT162B2 cannot be completely ruled out considering the temporal relationship. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | Tested positive for COVID-19; CT showed increased infiltrates 10-15%; Dehydration/Dehydrated; Chills; Tested positive for COVID-19; Hypotensive; Achy; Severe achy cramps/Severe cramps all over body; This is a spontaneous report from a contactable nephrologist (patient himself). This 78-year-old male patient received the first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE, Lot number EK5730), via an unknown route, on 17Dec2020 at single dose for COVID-19 immunisation. Age at vaccination was 78-year-old. The patient was diabetic and hypertensive. Additional medical history included hyperlipidaemia. No relevant concomitant medications were provided. On 18Dec2020, the patient developed severe achy cramps/severe cramps all over body. On 19Dec2020, the patient developed achy. On 20Dec2020, the patient was dehydrated and hypotensive, he had also chills. On unknown date, blood pressure was down to 76/50. His symptoms for COVID were severe achy cramps, hypotension, and dehydration. On 20Dec2020, COVID-19 test was positive. On 21Dec2020, the patient was given monoclonal antibodies. A computerized tomogram (CT) of the lungs was performed on 21Dec2020 and it was ok. A week later (Dec2020), he had a repeat CT which showed increased infiltrates of 10 to 15%. He then started on dexamethasone, apixaban (ELIQUIS) and the rest of the things. He had a repeat CT on 05Jan2021 which showed resolution of the infiltrates; most of the lesions went gone. CT results had improved significantly. The patient underwent a second COVID test a week ago which was still positive. He had a third COVID on 06Jan2021, but results were not available yet. The patient queried if he can proceed with second dose planned on 07Jan2021 or if he should wait. The clinical outcome was recovered for the event ‘severe achy cramps/severe cramps all over body’ on 19Dec2020, for ‘dehydration/dehydrated’ on 20Dec2020, for ‘chills’ on unknown date in Dec2020, for ‘achy’ on 30Dec2020, for ‘hypotensive’ on 20Dec2020; the outcome of the event ‘CT showed increased infiltrates 10-15%’ was recovering; the outcome for ‘Tested positive for COVID-19’ was unknown. The reporter considered the events ‘achy’ and ‘severe achy cramps/severe cramps all over body’ serious because causing disability; the events ‘tested positive for COVID-19’, ‘dehydration/dehydrated’, ‘chills’ and ‘hypotensive’ were considered medically significant. The reporter considered the events ‘Tested positive for COVID-19’, ‘CT showed increased infiltrates 10-15%’ and ‘dehydrated/dehydration’ unrelated to BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE).; Sender’s Comments: A possible contributory effect of suspect BNT162B2 on reported events cannot be excluded. Case will be reassessed when new information is received. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to regulatory authorities, Ethics Committees, and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | “Hypokalemia; Hypomagnesemia; Hypocalcemia; Hemoglobin dropped little bit; Tetany; Muscle cramps; This is a spontaneous report from a contactable healthcare professional, a physician assistant. A 50-year-old female patient received the first dose of the BNT162B2 (PFIZER-BIONTECH COVID-19 mRNA VACCINE; Lot Number: UNKNOWN), via an unspecified route of administration on 18Dec2020 (at the age of 50-years-old) as a single dose for COVID-19 immunization. Medical history included tachycardia, tension headache, and glucose tolerance impaired. Concomitant medications included rosuvastatin calcium (CRESTOR), gabapentin (MANUFACTURER UNKNOWN), and metoprolol (MANUFACTURER UNKNOWN). On 18Dec2020, the patient experienced hypokalemia, hypomagnesemia, hypocalcemia, tetany, muscle cramps, and hemoglobin dropped little bit. The clinical course was as follows: The patient received the vaccine and about 30 minutes later she started to “”feel bad””. She went to the urgent care and about an hour after they took her to the emergency room (also reported as hospital). She had a complete blood count, complete metabolic panel, and magnesium level done on the 18th, 21th, 23rd, 26th, 28th, 30th of Dec2020 and then again on 04Jan2021. On 18Dec2020, her initial lab test showed potassium: 2.6, hemoglobin: 10.2, magnesium: 1.2, and calcium: 5.7. The physician assistant reported that a potassium of 2.6 was critically low and a hemoglobin was 10.2 was about normal for the patient. She received oral medications of potassium 20 mEq twice a day and calcium and magnesium supplements once a day. On 04Jan2021, lab data showed: potassium: 4.2, magnesium: 2.1, calcium: 9.5 and hemoglobin: 14.5. Per the reporting physician assistant, the above levels went back to normal but stated she had to be supplemented to get back to that point. The clinical outcomes of the hypokalemia, hypomagnesemia, hypocalcemia, and hemoglobin dropped little bit were recovered on 04Jan2021; while that of the tetany and muscle cramps, were unknown. The physician assistant assessed the events as related to the suspect vaccine. The lot number for the vaccine, BNT162B2, was not provided and will be requested during follow up.; Sender’s Comments: A possible contributory effect of suspect BNT162B2 on reported events cannot be excluded. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to regulatory authorities, Ethics Committees, and Investigators, as appropriate.” | |
| COVID19 VACCINE (COVID19) | died; This is a spontaneous report from a non-contactable consumer via a Pfizer-sponsored program. A patient of unspecified age and gender received bnt162b2 (PFIZER-BIONTECH COVID-19 VACCINE, via an unspecified route of administration on an unspecified date at single dose for covid-19 immunisation. The patient medical history and concomitant medications were not reported. It was reported the patient was a doctor, died after the vaccine with no apparent disease. It was not reported if an autopsy was performed. No follow-up attempts are possible. Information about lot/batch number cannot be obtained.; Reported Cause(s) of Death: Unknown cause of death | |
| COVID19 VACCINE (COVID19) | COVID-19 positive and admitted to hospital 6 days post-vaccination | |
| COVID19 VACCINE (COVID19) | Received first dose Moderna COVID vaccine on 12/28/2020; on that same day 12/28 he noticed a dry cough; on 1/1/2021 he reported fever, chills, body aches, Headache, sinus congestion. On 1/1/2021 he tested positive for COVID-19, he reported being with a family member on Christmas who had COVID symptoms. On 1/11/2021 he required hospitalization for COVID-19 pneumonia | |
| COVID19 VACCINE (COVID19) | on 1/8/2021 17:30 patient taken to ER, cerebellar hemorrhage, stroke, aneurysm |
| COVID19 VACCINE (COVID19) | Following the first COVID vaccine dose on Dec/18/2020, I had headaches that started on the third day and ended on the tenth day. The headaches were usually light, unilateral, and alternating from one side to the other. I was usually functional except on the fourth and seventh days where the headaches were moderate to severe, and I took naps to help with the headaches for those two days. I have never had an issue with headaches before, and these symptoms were a new experience for me. I did not take any medications as treatment for the headaches. Following the second COVID vaccine dose on January/7/2021, I felt fatigue and generalized muscle aches within six to twelve hours, and these symptoms lasted for two days. On January/10/2021, when I woke up that morning I again felt light, unilateral, and alternating headaches. In addition, I noticed that I was unable to move the left side of my face. I felt moderate tingling sensations associated with the distribution of the paralysis. When I looked in the mirror, I could quite noticeably see asymmetry in my face. I immediately went to the emergency department at the hospital where my primary care doctor is located. I was kept in the hospital into the next day for observation. After evaluation by a neurology team and an MRI, I was provided with the diagnosis of Bells Palsy. I have never previously been diagnosed with Bells Palsy, and I have never previously had a hospital stay before. The doctors prescribed medications which I am currently taking. As of today January/12/2021, the symptoms have had some improvement, but the symptoms still continue. | |
|---|---|---|
| COVID19 VACCINE (COVID19) | Beginning at 6:30 am on 1/8, two days following vaccination, the individual was driving to work and began to experience nausea while at a traffic light. He pulled over and threw up. Once he got to work he vomited again. He drove to another facility for work and then threw up 3 or 4 more times. He began experiencing hot flashes at this time. He went to the hospital and was seen in the emergency room. He threw up several more times in the ER and stated that his heart was racing and that he had diarrhea. The emergency room gave him medications for the nausea and he stayed there for 2 or 3 hours. He began to feel better and was discharged to home. | |
| COVID19 VACCINE (COVID19) | Immediately developed intense burning that progressed over the next 30 minutes and continued to burn for 3 days. The next day progressive extreme fatigue last 4 days. felt like it was going to pass out. full on body pain, dizzy and lightheaded. need assistance to get to the walk, intense headache. | |
| COVID19 VACCINE (COVID19) | Very large, reddened, tender area at injection site (like the size of an angry red egg), and fever of 103.2. | |
| COVID19 VACCINE (COVID19) | GIVEN ON 12/23. SORENESS FELT ON LEFT ARM SITE THE NEXT DAY, WITH DECREASED MOBILITY AND STRENGTH DUE TO SEVERE SORENESS AND PAIN. PAIN PROGRESSIVELY LESSENED BUT THE SORENESS STILL VERY APPARENT. ON 1/5, SPOKE TO PRIMARY MD, XRAY ON LEFT SHOULDER DONE ON 1/6. ON 1/7, STIFFNESS WITH SEVERE PAIN UPON MOVEMENT NOTED ESP IN THE MORNING. ULTRASOUND WAS DONE ON 1/8, NOTED BICEPS TENOSYNOVITIS. ORTHOPEDIC SURGEON SEEN ON 1/11, W DX OF ADHESIVE CAPSULITIS, SUGGESTED FOR PT FOR NOW, AND OFF WORK, AND TO BE FOLLOWED UP ON 2/1 BY SAME ORTHO SURGEON. WILL NOT OFFER CORTISONE SHOT FOR NOW AS IT MAY COMPROMISED OR WEAKEN IMMUNE RESPONSE, IN WHICH MY SECOND COVID SHOT DUE ON THE 15TH OF JANUARY. FIRST APPT FOR PT ON 1/15 | |
| COVID19 VACCINE (COVID19) | Sudden hearing loss right ear accompanied by tinnitus | |
| COVID19 VACCINE (COVID19) | Sudden hearing loss right arm accompanied by tinnitus | |
| COVID19 VACCINE (COVID19) | Patient presented on the morning of 1/10/2021 with swollen lips and hives. Vaccination took place on Wednesday January 6th, 2021. | |
| COVID19 VACCINE (COVID19) | Patient had mild bilateral knee and hip pain 1 month ago, then she received her 1st dose of COVID vaccine on 12/14/20. Her joint pain worsened then on 1/4/21 she received her 2nd dose of COIVD vaccine on 1/4/21 and then her pain increased in spread up to her shoulders. She was seen at immediate care on 1/11/21 because her joint pain had worsened throughout and her shoulder pain was making it difficult to raise her arms above the level of her shoulders. Her pain was made worse w/ movement. | |
| COVID19 VACCINE (COVID19) | Woke up on 1/6/2021 with hot flashes, palpitations, dizziness and heart racing. Went to urgent care and they did an EKG which showed A-Fib, so I was sent to the ER and from there, I was transferred to an ICU at a different facility . I stayed until 1/8/2021. No cause was found and no history of A-Fib or family history. | |
| COVID19 VACCINE (COVID19) | immediate tingling of lips, followed by fullness of posterior oropharynx, hoarseness and pruritus | |
| COVID19 VACCINE (COVID19) | “Fever (103-104 oF) and 4″”x1″” red, swelling area around site of injection. Pt states she received an IV.” | |
| COVID19 VACCINE (COVID19) | Acute approximate respiratory failure secondary to acute COPD | |
| COVID19 VACCINE (COVID19) | first day after shot, nausea, body aches, 2nd day Sunday headache, Monday 5 am woke up itching, then 9 am hives everywhere, trouble breathing, anaphylaxis, went to ER, got epi X 2, solumedrol, benadryl, pepcid, then still with hives, tachycardia, dyspnea, iv fluids were influsing and epi drip started, went to ICU | |
| COVID19 VACCINE (COVID19) | Back pain, bilateral PE and DVT | |
| COVID19 VACCINE (COVID19) | “Patient states that she received her second vaccination and in the hours after she had flu-like symptoms. Then over the next few days, she started to notice tingling and a “”prickly”” sensation in various areas. This progressed to symmetric BLE weakness which started in her feet and had reached to just above her knees bilaterally time and she arrived. The weakness had progressed to her hips. She also noticed weakness in her arms and they are easily fatigued. She is able to walk but it takes much effort.” | |
| COVID19 VACCINE (COVID19) | coughing, flushing, cyanosis, diaphoresis | |
| COVID19 VACCINE (COVID19) | Within 3 minutes of receiving vaccine felt flush and throat swelling, responded to Epi Pen and Benadryl p.o. EMS took him to ED where he remained several hours receiving 1 liter NS 125 mg solumedrol IV, discharge with 4 days of prednisone 40 mg daily and a prescription for an Epi Pen. As of 1.12 he is totally okay with no after effects. | |
| COVID19 VACCINE (COVID19) | First Day after the injection I had a headache and nausea the entire day into the next day. The second day I still had the headache and the nausea. I work overnights. When I awoke in the afternoon, my throat was closing up. It was hard to swallow and I struggled to breath. I immediately drank liquid Benadryl and called my doctor in the morning. | |
| COVID19 VACCINE (COVID19) | -0715 vaccine administered. -0735 started to feel dizzy/off and right side of tongue felt like it was mildly swelling and itchy. -0735 asked to have blood pressure taken as know when I am having anaphylaxis my blood pressure escalates. -0740 took blood pressure and it was 141/86 in right arm. Normal is 110s/60s-70s. No anxiety feelings. -0740 throat started to have increased mucous production. Had the tickle and tightness in throat. Asked and received 25mg Benadryl with cup of water. -0742 started clearing throat frequently and slight cough. Knew it was anaphylaxis and told the team I need to go to the ER. Asked for additional 25mg Benadryl. Also took 20mg Famotidine and 2 puffs Albuterol inhaler–this is my prescribed anaphylaxis routine. Had Epipens on standby. -0743 put on O2 saturation monitor and watched O2 drop into 90-92 range. Asked for epipen on standby as I know when I need to start it. Didn’t want to take that when I knew I was about to get it in the ER and knowing self hadn’t progressed that far. Felt chest tightness and shortness of breath. Voice started becoming hoarse. -0800 EMS arrived (delay as team didn’t know if they were supposed to call 911 or a Code–they chose EMS even though in hospital). Then staff at COVID vaccine clinic kept emphasizing need to go in ambulance while EMS and self fought to go through hospital (much quicker route). Finally cleared to go through hospital to ER. To get some air via breathing in had to sit up leaning forward. Voice completely hoarse by this time. -About 0817 arrived to ER bay. At this time, frequently coughing and cough started to sound stridorous. Difficulty getting breaths in. Had chest pain near heart. Greeted by MD, 2 RNS, and technician. -0819 received IM epinephrine. Attached to 5 lead EKG monitoring and O2 monitoring. Blood pressure done again. Higher than previous. -About 0821 had working IV (previous two attempts failed as veins were constricting). Given IV Solumderol. Started bolus of 1L Normal Saline. -Not sure how long after by cough subsided, increased mucous production subsided, as well as hoarseness decreased. -Held for observation for 2hours (would be longer if not resolved). – Discharged around 1015. At this time, hoarseness almost all gone. Minimal throat clearing. Cough resolved. -Prescribed epipen inhalers (mine expired) and Prednisone. Prednisone is PRN for mild breathing difficulties if it starts again tomorrow 1/13/21. -At 1400 took 50mg Benadryl and 20mg Famotidine as previously prescribed for anaphylaxis maintenance. Will continue this as previously prescribed every 6hours until symptoms stay resolved. -Made follow up appointment with Primary Care Physician per protocol | |
| COVID19 VACCINE (COVID19) | Immediately after she felt faint, heart rate 121, felt faint again bp 62/33. Was taken to the ER, within a half hour, she fully recovered. Vitals went back to normal. | |
| COVID19 VACCINE (COVID19) | Hospice Resident received first Covid 19 vaccine dose on 1/6/21. 1/7/21 resident had decreased appetite noted in am but ate 100% of meal at dinner. 1/9/21 resident had decreased appetite with emesis x 2, loose BM x 2. Call placed to hospice. 1/10/21 5:44 am resident able to take HS meds, ingest 2 cups of shake. No emesis or loose stool noted. 12PM nurse noted resident not eating meals but ingesting milkshake and medications without any problems. Hospice contacted for change in condition. 1:00 pm hospice ordered Phenergan 12.5 mg Q 6 hrs PRN. Labs to be drawn 1/11/21. Hospice notified POA. 1/11/21 12:24am Resident had blood in stool. Resident denies any pain, on 2L of O2 for comfort. | |
| COVID19 VACCINE (COVID19) | Severely dizzy, left hand totally numb but painful, cold to touch. Felt better before she got to ER. | |
| COVID19 VACCINE (COVID19) | Patient vaccinated on 12/28. Approximately one day later, develops cough and on azithromycin x 1 week. On 1/3, patient develops left-sided weakness and aphasia. Taken to the hospital, tested COVID+, required intubation — acute hypoxic respiratory failure secondary to COVID – on H&P. Patient died on 1/4/21 at 7:20am. | |
| COVID19 VACCINE (COVID19) | Attempting to confirm which COVID 19 vaccine was given (Moderna or Pfizer). They did not send the record when they sent the patient to General ER the next am. Did not answer the phone. | |
| COVID19 VACCINE (COVID19) | Started to feel lightheaded, weak, faint like I was going to pass out, heart rate increased, confusion, trouble speaking, brought to the ED, throat started to swell and started having thick spit and clearing my throat excessively. Diagnosed as anaphylaxis. | |
| COVID19 VACCINE (COVID19) | within 1 hr post-vaccine on 1/7 I had a mild headache that resolved with Tylenol. At about 12 hours post-vaccine I developed nausea, fever (100.4) and chills and secondary to this had poor sleep. The next day I took scheduled alternating Tylenol & ibuprofen during the day and then overnight 1 episode of chills that woke me up. no events Saturday or Sunday. Then Monday 1/11 in the early morning I started to develop a rash on my b/l elbow and right foot 3rd toe. I applied mometasone topical cream to these locations. while at work the rash extended down both forearms then by 5pm it was on both hips and extending along both legs. I applied Benadryl cream to the most irritated sites and took PO Benadryl 50mg at bedtime and again at 1am when the itching woke me up. I repeated Benadryl 25mg at 8am. The rash seems to be getting better on the arms but then by noon I had a new breakout on my neck and face. I took Benadryl 50mg at 1pm. The rash continued to have a rapid progression over the next hour and resulted in angioedema with my throat swelling, lips puffed and numb and eye swelling. I was injected with an epi pen and sent to the ED where I received PO prednisone, famotidine, and Benadryl. The face/neck rash then greatly improved and I was sent home on prednisone 40mg daily for 3 days. | |
| COVID19 VACCINE (COVID19) | Blurred vision, difficulty breathing (pale skin/blue lips), profuse sweating, muscle fatigue, headache. This lasted about 15 minutes. Until severity went down. Followed by 20 minutes of profuse sweating and headache. I thought I was going to die | |
| COVID19 VACCINE (COVID19) | Sudden cardiac death | |
| COVID19 VACCINE (COVID19) | Death; Malaise; Vomiting; This is a spontaneous report received from a contactable physician from the Regulatory Agency (RA). The Regulatory Authority report number is GB-MHRA-WEBCOVID-20210105172532, Safety Report Unique Identifier GB-MHRA-ADR 24558660. An 81-year-old female patient received bnt162b2 (BNT162B2) (lot# EJ1688), via an unspecified route of administration, on 30Dec2020, at single dose, for COVID-19 immunisation. Medical history included vascular dementia (advanced dementia), dementia Alzheimer’s type (vascular and Alzheimer’s mixed dementia), oral intake reduced (patient known to not be eating or drinking), fluid intake reduced, (patient known to not be eating or drinking), general physical health deterioration (patient known to be declining); all from an unknown date and unknown if ongoing. Concomitant medications were not reported. The patient experienced death on 03Jan2021, malaise on 01Jan2021 with fatal outcome, vomiting on 01Jan2021 with fatal outcome. It was reported that 48 hours after vaccination the patient became unwell, vomited and then died on 03Jan2021. The patient underwent lab tests and procedures which included COVID-19 virus test: negative on 27Dec2020. Patient has been not tested positive for COVID-19 since having the vaccine. It was not reported if an autopsy was performed. It was not known whether vaccine caused reaction. No follow-up attempts are possible. No further information is expected.; Reported Cause(s) of Death: Malaise; Vomiting; Death | |
| COVID19 VACCINE (COVID19) | breathless on exertion; This is a spontaneous report received from a contactable other health professional received from the United Kingdom’s Medicines and Healthcare products Regulatory Agency (UK-MHRA). The regulatory authority report number is GB-MHRA-ADR 24561910, other case identifier number: GB-MHRA-WEBCOVID-20210106094618. An 80-years-old male patient received bnt162b2 (PFIZER-BIONTECH COVID-19 VACCINE, Batch/lot no: EJ1688), via an unspecified route of administration on 30Dec2020 single dose for covid-19 immunisation. Medical history included Bowen’s disease, basal cell carcinoma, chronic kidney disease and essential hypertension, all unknown if ongoing. Concomitant medication included alfacalcidol (unknown manufacturer), amlodipine (unknown manufacturer), atorvastatin (unknown manufacturer), clopidogrel (unknown manufacturer), prazosin (unknown manufacturer), sodium bicarbonate (unknown manufacturer), folic acid (unknown manufacturer), furosemide (unknown manufacturer). The patient experienced breathless on exertion on 02Jan2021. The patient died on 02Jan2021 due to the event. The patient underwent lab tests and procedures which included sars-cov-2 test: no – negative covid-19 test on unknown date. It was not reported if an autopsy was performed. No follow-up attempts possible. No further information expected.; Reported Cause(s) of Death: Dyspnoea exertional | |
| COVID19 VACCINE (COVID19) | On 1/5/2020, I, the patient woke up at 3:30 with sharp boring epigastric pain. Progressed to include nausea. On evaluation in the ED was diagnosed with acute pancreatitis. Prior hx includes 2 episodes with hospitalization for gallstone pancreatitis in 2016. Subsequently had laparoscopic cholecystectomy in 2016. Last alcoholic beverage prior to 2020 ED presentation was 3 weeks prior. No tobacco or drug use. | |
| COVID19 VACCINE (COVID19) | The patient presented to hospital on 1/6/2021 with a primary complaint of Fatigue (pt had covid vaccine yesterday. Now displaying increased weakness, blood in urine, increased confusion and urinated on herself today. Fever of 101.9 ) 79-year-old female presents to the emergency room with fatigue. The patient states yesterday she had the Moderna COVID-19 The patient states today she had a temperature 101.8¦ prior to arrival. Her son noted that she was having episodes of urinary frequency and also that she was profoundly weak and fatigued. They denied falling or hitting her head. The patient also states she been having nausea vomiting with diarrhea. | |
| COVID19 VACCINE (COVID19) | Vaccine was given on 12/23/20 on 01/07.21 went to get a routine physical and received an emergency call that my pallet blood count was around 10K instead of 150k. Was instructed to go to the ER asap. However didn’t received the message until the next morning at 6:30am. Was checked into the E.R. , given sterioids, pallets, hemogolibin. Stayed overnight in the hospital was able to leaved the next day around 1pm with pallets at 47k. | |
| COVID19 VACCINE (COVID19) | swelling at the injection site, pain, headache, feeling like numb, change in the taste, fever the 1st day x2, body ache, lower back pain. | |
| COVID19 VACCINE (COVID19) | Three hours after receiving COVID 19 vaccination, Patient oxygen level decreased to a critical level and went into cardiac arrest. Staff performed full code but was unable to bring back patient from cardiac arrest. | |
| COVID19 VACCINE (COVID19) | 2230 feeling of unease, body aches, site arm tingling, general mild aches 0220 awoke from sleep choking, having difficulty breathing, felt very SOB, worse with exertion or trying to speak, great difficulty swallowing and speaking even in brief words. Took 50mg of Benadryl PO and went to the ED, about a 15 minute car ride. Had tingling and numbness of the tongue and back of throat by arrival but still able to breath with focus. Exertion of just walking into the ED greatly increased the SOB. Was triaged, Benadryl starting to help, was able to speak a little better, 3-4 words without too much SOB caused. Was walked to a room, SOB milder with that exertion. Seen by Dr. Given IV Sol-u-Medrol and 50mg Benadryl. Was observed on cardiac monitor/Q15VS for a few hours and discharged home around 5:30. Given Rx of Prednisone 20mg -3tabs x2 days, 2tabs x5 days all once a days and told to take 50mg of Benadryl Q4H for the next 24 hours at least and to return prn. I did need to stay on Benadryl, as the Sol-u-Medrol wore off some of the swelling in thr throat did return but not severe, Benadryl did help, along with taking my Asthmnex I already had. I also continued my normal HS antihistamines. I had SOB on exertion, progressively better from the 6th-10th with it mostly resolved to yesterday. Body aches have continued but also progressively better. Yeasterday1/12/21 the Rx of prednisone was completed and I did have some mild swelling /tingling in the throat/face/mouth return in the evening, took Benadryl 50mg again and inhaler used. I have an appointment today to seek further care at my primary doctor’s office. Asthmnax used again this morning as well, only mild tightness in the throat currently with mild body aches this whole time. | |
| COVID19 VACCINE (COVID19) | Systemic: Fever-Severe, Systemic: hypotension, dyspnea-Severe; symptoms lasted 1 day | |
| COVID19 VACCINE (COVID19) | Patient day after vaccination had fever, chills, headache and malaise but recovered the next day. starting four days after vaccination started to have left neck swelling . Of note day prior vaccination had left wisdom teeth removal and maxillary root canal. 1 week after vaccination and 8 days after oral procedures worsening left neck swelling, trouble swallowing’s and change voice found resulting left neck 3x2cm necrotic neck abscess and significant neck inflammation. Patient was seen by ENT and started on IV Unysan and admitted for airway monitoring due to swelling with improvement over next 48 hours. | |
| COVID19 VACCINE (COVID19) | 12/23/2020-RECEIVED VACCINE AT 9:52 AM. REPORTS NOT FEELING WELL IN THE AFTERNOON, LIGHTHEADED AND DIZZY, THROBBING HEADACHE. CRAMPING/ACHING IN BACK OF CALVES. 12/24/2020 CONTINUED WITH DIZZINESS, THROBBING HEADACHE, BLOOD PRESSURE ELEVATED, CHEST PRESSURE 8:00 PM REPORTED FEELING THAT SHE WAS GOING TO DIE, WENT TO LAKE CITY MEDICAL CENTER- BLOOD PRESSURE ON ARRIVAL 184/101 HR. 117. GIVEN NITRO AND MEDICATIONS. ADMITTED, DISCHARGED 48 HOURS LATER ON 12/26/2020. DISCHARGED ON BLOOD PRESSURE MEDICATION 12/27/2020-LESS THAN 24 HOURS AFTER BEING DISCHARGED SHE WAS READMITTED TO MEDICAL CENTER WITH SAME SYMPTOMS. 36 HOURS AFTER ADMISSION, TRANSFERRED TO MEDICAL CENTER. FOR CARDIC WORKUP AND HIGHER LEVEL OF CARE. | |
| COVID19 VACCINE (COVID19) | Became dizzy, headache, felt like she was going to faint, rigors and a temperature of 101.5. Next morning she had upset stomach, feeling dizzy, thought she was going to faint and a headache. | |
| COVID19 VACCINE (COVID19) | Had Covid Vaccine AM of 1.8.2021. Woke up with Nausea, Vomiting X 4, Chills the following AM. Presented to ED. Found to be hypotensive during ER. Nausea Vomiting resolved. Tx with fluid replacement and dismissed home instructed to monitor BP. B/P dropped again at home returned and admitted to hospital. | |
| COVID19 VACCINE (COVID19) | Site: Pain at Injection Site-Medium, Systemic: Generalized Body Aches -Severe, Systemic: Headache-Severe, Systemic: Severe drop in blood pressure, pulse, and o-sat-Severe | |
| COVID19 VACCINE (COVID19) | Patient reported symptoms started ~15 mins s/p dose. Symptoms reported LUE and LLE numbness/tingling, and notable tachycardia | |
| COVID19 VACCINE (COVID19) | NauseaVomiting, HYPERtension, Tachycardia, throat swelling Narrative: Went home after vaccine and starting vomiting, throat swelling. HR and BP elevated Went to ER was given beta blockers, in hospital for observation. | |
| COVID19 VACCINE (COVID19) | “Patient received vaccine on 1/8/2021. On 1/9/2021 I checked on patient via phone for symptoms or problems and he reported none but mild soreness at injection site. On 1/10/2021 family friend called me to tell me that patient had expired at about 8:00 pm. Patient reportedly complained of “”pain”” unspecific and collapsed at home. Hospital reportedly told family that it appeared to be a “”heart attack””.” | |
| COVID19 VACCINE (COVID19) | I was being monitored for 15 mins since I was allergic to tree nuts stayed 20 mins. I started feeling dizzy and exp nausea lasted 20 sec. After about 12 hrs later I woke up had difficult swallowing felt like something in my throat. I went to ER received a dose pack, Benadryl and steroid injection. | |
| COVID19 VACCINE (COVID19) | Jan 1st, patient had a seizure after breakfast. Temperature was 98.5 Pulse 95. B.P. 160/70 Two hours later another seizure at the home, with emesis, Sent to hospital at 2pm. Focal Status elepticus. Seizure activity along with continuous persistent activity with emesis throughout the 12 hour time in the ED. Seizure during the CT scan. | |
| COVID19 VACCINE (COVID19) | severe stabbing-shooting lower back pain; severe stabbing-shooting lower back pain that radiated to both legs; Pricking, pins and needles sensations in the hands and feet; numbness; weakness to both legs but mostly the right leg; Coordination problems, unsteadiness; Coordination problems, unsteadiness; This is a spontaneous report from a contactable nurse (patient). A 39-year-old male patient received first dose of bnt162b2 (PFIZER-BIONTECH COVID-19 VACCINE, Batch/lot number: EL0140), intramuscular on 20Dec2020 08:00 at single dose at left arm for covid-19 immunization. Medical history included hypertension from an unknown date and unknown if ongoing. The patient’s concomitant medications in two weeks included multivitamins. Patient didn’t receive any other vaccines within 4 weeks prior to the COVID vaccine. The patient was not diagnosed with COVID-19 prior to vaccination. No known allergies to medications, food, or other products. On 22Dec2020 at approximately 19:15, the patient experienced sudden onset of severe stabbing-shooting lower back pain that radiated to both legs. Pricking, pins and needles sensations in the hands and feet. Coordination problems, unsteadiness, numbness, and weakness to both legs but mostly the right leg. The patient underwent lab tests and procedures post-vaccination which included nasal swab for covid test: negative on 30Dec2020 (Antigen Test). Adverse events resulted in doctor or other healthcare professional office/clinic visit, emergency room/department or urgent care, disability or permanent damage. Patient received pain medication, steroid dose pack, MRI (pending), and physical therapy (pending) as treatment. Outcome of all events was not recovered.; Sender’s Comments: Based on the compatible temporal association, a contributory role of vaccination with BNT162B2 in the onset of the events cannot be excluded. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | high blood pressure within 15 min post vaccination. BP 180/94/BP rose to 202/110; This is a spontaneous report from a contactable nurse. An adult female (age:18-64 Years) patient (pregnant: No) received first dose of BNT162B2 (Pfizer), intramuscularly in left arm on 05Jan2021 10:30 at single dose for COVID-19 immunization. The patient’s medical history was not reported. The patient was received other concomitant drugs. The patient experienced high blood pressure within 15 min post vaccination. BP (blood pressure) 180/94 rose to 202/110 on 05Jan2021 10:45. The event resulted in doctor or other healthcare professional office/clinic visit, Emergency room/department or urgent care. The events were reported as non-serious. Recipient experienced high blood pressure within 15 min post vaccination. Went to hospital where her pressure was 202/110, vaccine given on 05Jan2021 at 10:30 am, by 10:45 am BP was 180/94, recipient refused hospitalization. Sister took her home but made her go to the hospital where her BP rose to 202/110. IV (intravenous) steroids given. The outcome of event was resolved in Jan2021. The patient didn’t receive any other vaccines within 4 weeks prior to the COVID vaccine. The medications the patient received within 2 weeks of vaccination: Protein drink. Unknown whether was the patient diagnosed with COVID-19 prior to vaccination. Unknown whether the patient been tested for COVID-19 Since the vaccination. Unknown allergies to medications, food, or other products. Information on the lot/batch number has been requested.; Sender’s Comments: Based on temporal association, a possible contributory role of BNT162B2 cannot be excluded for reported event hypertension. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | First dose on 16Dec2020, second dose on 05Jan2021; Tinnitus; constant ringing in ears; louder this time; This is a spontaneous report from a contactable nurse (patient). A 51-year-old female patient (no pregnancy) received bnt162b2 (PFIZER-BIONTECH COVID-19 VACCINE), with first dose (lot number: EH9899) on 16Dec2020 via intramuscular route, with second dose (lot number: EK4176) on 05Jan2021 via an unspecified route of administration, both at single dose for covid-19 immunization. Medical history included allergies: penicillin. Concomitant medication included famotidine, ibuprofen (DUEXIS), iron (IRON) and multivitamins, all received within two weeks of vaccination. On 17Dec2020, the patient experienced tinnitus, constant ringing in ears that started 24 hours after first vaccine, lasted about 2-3 days and went away. Ringing in ears started again and is louder this time on 06Jan2021. Facility where the most recent COVID-19 vaccine was administered was in hospital. The patient did not receive any other vaccines within 4 weeks prior to the COVID vaccine. No treatment received for the adverse event. Prior to vaccination, the patient was not diagnosed with COVID-19. Since the vaccination, the patient had not been tested for COVID-19. The event was identified as serious and resulted in disability or permanent damage. The outcome of event tinnitus was not recovered. The outcome of the other event was unknown.; Sender’s Comments: Based on the compatible temporal association with positive rechallenge result, the Company considers the event tinnitus is possibly related to vaccination with BNT162B2. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | left side will blur; Left side of face was sagging/ water leaking out of mouth/Progressive weakness on left side of face/ Swelling on lower left mandible/ diagnosed with Bell’s Palsy.; Eye tearing; This is a spontaneous report from a contactable nurse who reported for himself. A 61-year-old male patient received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE; Batch/lot number: EK5780) in left arm on 26Dec2020 at 08:30 at single dose for covid-19 immunisation (worked in surgical ICU and was over 61 years old). Medical history included Pre-diabetic. Family history included: mother died; mother’s side had colon cancer and grandparents and uncles had cardiovascular diseases.Concomitant medication included exenatide (BYDUREON), amlodipine besilate (NORVASC), omeprazole (PROTONIX), hydrochlorothiazide, lisinopril and pneumococcal vaccine on 08Dec2020 and tetanus vaccine on 08Dec2020. It was reported that on 31Dec2020 at 07:30, the patient had eye tearing and water leaking out of mouth, left side of face was sagging, swelling on lower left mandible (eye tearing was first, as reported); on 31Dec2020 he also experienced progressive weakness on left side of face; on 02Jan2021 the patient was diagnosed with Bell’s Palsy. Then on an unknown date, left side will blur occurred. All events required emergency room visit and physician office visit. Diagnosis of Bell’s Palsy and event eye tearing were serious per disability; left side will blur was non serious. Patient described the events as follows: on 31Dec2020 he was brushing teeth and noticed the water was going everywhere. Left side of face was sagging, noticed some swelling and thought it was from a bug bite. He wasn’t sure if it was a stroke or not. In the morning of 01Jan2021 noticed it was progressively causing a problem. Days before noticed tearing of left eye (as reported). On 31Dec2020 before midnight, something felt wrong. He saw four cases on clinical trial with similar side effects (he clarified he had no patient information for the four patients mentioned with similar side effects from Pfizer Clinical trial. He saw this information from a article; stated four from Pfizer and Moderna). In the morning of 02Jan202, he went to Emergency Room (ER) and was diagnosed with Bells Palsy. He was given prednisone 20mg to take 3 times by mouth every day for 5 days, tetracycline 100mg, at 1 capsule by mouth twice a day for 10 days and methylprednisolone (SOLU MEDROL; Lot: 9945776;Exp: Nov2021) 4mg dose pack, started with 6 tablets first day. It was told by doctor it might cause tick problems. He was waiting for results. On 04Jan2021 went to family doctor and more blood work was taken. Because he was taking prednisone, noticed his sugar was up a little bit (date unspecified). It was prescribed Glitizide extended release, 2.5mg one tablet twice a day with breakfast. Patient was checking sugar every 6 hours. It was also prescribed Acyclovir 400mg one tablet orally five times per day for 10 days. 08Jan2021 is last day of prednisone 5 day dose and will follow up with methylprednisolone tablets. Patient had an appointment with a neurologist on 13Jan2021. Patient was still having symptoms. It was really hard for him. Not hard to swallow. Face was still drooping. Eyes were still tearing. Could not work with eyes tearing all of the time. Needed to be alert. When driving, had to focus on the right side because his left side will blur. He had to chew only on the right side because food will be left behind in between his cheeks and gums. If he drank through a straw, he had to cover the left side of his lips so he was able to suck out fluids. He thought symptoms were progressively getting worse, he didn’t see much improvement. He clarified swelling was on lower part of mandible on left side. It was slightly bigger than right. When looking at face, the lines on his forehead on the left side were down. If he smiled he cannot raise his left eye brow, when before the COVID-19 vaccine he could. Noticed left side of nose was lower than the right. Cannot raise left side of lips. Outcome of the event Eyes tearing and Bell’s Palsy was not recovered; outcome of the other event was unknown. Information on the batch number has been requested.; Sender’s Comments: Based on available information, a possible contributory role of the subject product, BNT162B2 vaccine, cannot be excluded for the reported events of Bell’s palsy, Lacrimation increased and vision blurred due to temporal relationship. However, the Bell’s palsy may likely possibly represent concurrent medical condition in this patient. There is limited information provided in this report. Additional information is needed to better assess the case, including complete medical history, diagnostics including head CT/MRI and viral serologies, counteractive treatment measures and concomitant medications. This case will be reassessed once additional information is available. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | she was dying as her blood pressure dropped to 70/40 and to come for a last visit; This is a spontaneous report from a contactable consumer. A 100-year-old female patient received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration on 02Jan2021 at single dose for COVID-19 immunization. Medical history included COVID in Dec2020, urinary tract infection (UTI), dehydration and Covid sickness (vomiting) (was treated earlier in month for UTI and dehydration from the Covid sickness (vomiting)). Known allergies: no. The patient’s concomitant medications were not reported. After testing positive in mid December to COVID and being declared Covid free on 30Dec by the nursing staff and in good health, with normal vitals and oxygen levels, the patient was given a vaccination on 02Jan2021. In the early evening the patient’s blood pressure dropped to 70/40 and the reporter was told to come for a last visit. The patient was sleeping comfortably. She did not wake up when spoke with her. No one expected her to make it through the night. The next morning she work up, ate breakfast, watched TV, got IVs and oxygen and her vitals improved significantly. Lab tests and procedures included blood pressure: 70/40 on 02Jan2021, oxygen levels: normal, COVID test: positive in Dec2020 (testing positive in mid December to COVID and being declare Covid free on 30Dec), vitals: normal; improved significantly. Facility where the most recent COVID-19 vaccine was administered: Nursing Home/Senior Living Facility. If the patient received any other vaccines within 4 weeks prior to the COVID vaccine: No. Prior to vaccination, was the patient diagnosed with COVID-19: Yes. Since the vaccination, has the patient been tested for COVID-19: No. AE resulted in: Life threatening illness (immediate risk of death from the event). Serious: Yes. Seriousness criteria-Results in death: No. Seriousness criteria-Life threatening: Yes. Seriousness criteria-Caused/prolonged hospitalization: No. Seriousness criteria-Disabling/Incapacitating: No. Seriousness criteria-Congenital anomaly/birth defect: No. Information about lot/batch number has been requested. | |
| COVID19 VACCINE (COVID19) | dizzy; itchy throat; coughing; swollen throat; This is a spontaneous report from a contactable pharmacist. A 21-year-old female patient received first dose of bnt162b2 (PFIZER-BIONTECH COVID-19 VACCINE, Batch/lot number: EL0142, NDC number: 59267-1000-1; Expiry Date: Mar2021), via an unspecified route of administration on 07Jan2021 11:20 at 0.3 mL, single at left arm for vaccination. Medical history included Cushing’s disease (recovering), bipolar disorder from an unknown date and unknown if ongoing. Concomitant medication included lamotrigine (LAMICTAL) for bipolar disorder. On 07Jan2021, looked like after receiving the vaccine about 30 minutes later patient was standing and felt like dizzy. About 15 or 20 more minutes, or 45-50 minutes after received injection, she felt like itchy throat, then itchy throat triggered coughing, then she felt like swollen throat. For the treatment on scene patient was administered 50mg of diphenhydramine hydrochloride (BENADRYL) and when she complained of swollen throat was administered epinephrine (EPI-PEN) 0.3mg injection to right thigh. Then they called EMS who transported her to the hospital where she was admitted to the hospital. Outcome of events was unknown. This report was considered as serious per caused/prolonged hospitalization. Causality: Cannot jump to that conclusion.; Sender’s Comments: Based on temporal association, the causal relationship between bnt162b2 and the events dizziness, throat irritation, cough and pharyngeal swelling cannot be excluded. The information available in this report is limited and does not allow a medically meaningful assessment. This case will be reassessed once additional information becomes available. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees, and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | patient passed away after receiving the Covid vaccine; This is a spontaneous report from a contactable nurse. An 81-year-old male patient received BNT162B2 (PFIZER-BIONTECH COVID-19 mRNA VACCINE), intramuscular into the right arm on 07Jan2021 at 0.3 mL, single for covid-19 immunization. There was no medical history and no concomitant medications. On 08Jan2021, the patient passed away after receiving the COVID vaccine. The patient died on 08Jan2021. An autopsy was not performed. Investigations indicate that unspecified labs were done, but nothing two weeks prior; no further details were provided. The patient received the first dose the day prior. The reporting nurse discussed it with the medical director, and he thought that he potentially passed away from the COVID vaccine. The relatedness of the event to the suspect vaccine was reported as related by the reporting nurse per The Agency. The batch/lot number for the vaccine, BNT162B2, was not provided and will be requested during follow-up .; Sender’s Comments: Based on the limited information available, it is medically not possible to make meaningful causality assessment, it is unlikely the vaccine could have contributed to the death of the patient based on the known safety profile. However case will be reevaluated when additional information is received during the follow-up The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate.; Reported Cause(s) of Death: Stated that the patient passed away after receiving the Covid vaccine | |
| COVID19 VACCINE (COVID19) | Patient received her vaccination on 1/12/21 administered by pharmacy*+. She expired on 1/12/21 an approximately 7:30pm. Resident did not have any adverse reactions and was a hospice patient. | |
| COVID19 VACCINE (COVID19) | “Patient was found “”acting abnormal”” on 1/9/2021 at 1215. VS HR 20-30’s. EMS activated. EMS arrived and patient was found pulseless in PEA/ asystole, CPR and ACLS initiated and then transported to the MC. Unsuccessful resuscitation and expired on 1/09/2021 at 1348. Clinical impression Cardiopulmonary arrest.” | |
| COVID19 VACCINE (COVID19) | Death; Vomiting; This is a spontaneous report from a contactable other health professional from the Regulatory Agency. The regulatory authority report number is GB-MHRA-ADR 24573192. An elderly female patient received the bnt162b2 (PFIZER-BIONTECH COVID-19 MRNA VACCINE; Lot Number: BJ1688 and EJ1688; as reported), via an unspecified route of administration on 05Jan2021 at 12:26 at a single dose for COVID-19 immunization. The patient’s medical history and concomitant medications were not reported. The patient had not had symptoms associated with COVID-19; and was not enrolled in the clinical trial. On 05Jan2021 at 12:51, the patient experienced vomiting (non-serious); 25 minutes post vaccine (had further vomiting episodes). On 07Jan2021 at 01:00, the patient experienced death; which caused death, and was medically significant. The patient had not tested positive for COVID-19 since having the vaccine. The patient underwent lab tests and procedures which included COVID-19 virus test: no-negative COVID-19 test on an unspecified date. The clinical outcome of the event, vomiting, was unknown. The clinical outcome of the event, death, was fatal. The patient died on 07Jan2021 at 01:00 due to unknown cause of death. An autopsy was not performed. No follow-up attempts are possible. No further information is expected.; Reported Cause(s) of Death: Death | |
| COVID19 VACCINE (COVID19) | thrombopenia; pulmonary embolism; neutropenia fever; This is a spontaneous report from a Pfizer-sponsored program . A contactable consumer reported for a patient that received BNT162B2 (PFIZER-BIONTECH COVID-19 MRNA VACCINE), via an unspecified route of administration on an unspecified date at a single dose for COVID-19 immunization. The patient’s medical history and concomitant medications were not reported. The patient experienced thrombopenia, pulmonary embolism and neutropenia fever on an unspecified date. The clinical outcome of thrombopenia, pulmonary embolism and neutropenia fever was fatal. The patient died on an unspecified date. It was unknown if an autopsy was performed. The batch/lot number for the vaccine, BNT162B2, was not provided and will be requested during follow-up.; Reported Cause(s) of Death: thrombopenia; pulmonary embolism; neutropenia fever | |
| COVID19 VACCINE (COVID19) | “Heart attack; This is a spontaneous report from a contactable consumer. An 82-year-old female patient received the first dose of bnt162b2 (PFIZER-BIONTECH COVID-19 MRNA VACCINE; Lot Number: and Expiration Date: Unknown), via an unspecified route of administration in the left arm on 05Jan2021 at 13:00 at a single dose for COVID-19 immunization; administered in doctor’s office/urgent care. The patient’s medical history and concomitant medications were not reported. It was unknown if the patient received any other vaccines within four weeks prior to the COVID vaccine. Prior to the vaccination, the patient was not diagnosed with COVID-19. Since the vaccination, the patient had not been tested for COVID-19. On 05Jan2021, the patient experienced heart attack; which resulted in death and was assessed as medically significant. The patient also experienced the associated symptoms of cold sweats, chest pain, shortness of breath. Therapeutic measures were taken as a result of heart attack, which included “”life saving measures”” by the paramedics performed upon arrival with no success. The clinical outcome of the event, heart attack, was fatal. The patient died on 05Jan2021 due to heart attack; as ruled by the paramedics. It was unknown if an autopsy was performed. The batch/lot numbers for the vaccine, PFIZER-BIONTECH COVID-19 MRNA VACCINE, were not provided and will be requested during follow up.; Reported Cause(s) of Death: Heart attack” | |
| COVID19 VACCINE (COVID19) | “Cardiac Arrest; Patient was found pulseless and breathless 20 minutes following the vaccine administration.; Patient was found pulseless and breathless 20 minutes following the vaccine administration.; This is a spontaneous report from a contactable other healthcare professional (HCP). A 66-year-old female patient (pregnant at the time of vaccination: no) received the second dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE, lot number: EL1284) via intramuscular at left arm on 11Jan2021 12:15 PM at single dose for COVID-19 immunization. Medical history included diastolic CHF, spinal stenosis, morbid obesity, epilepsy, pulmonary hypertension and COVID-19 (Prior to vaccination, the patient was diagnosed with COVID-19). The patient received medication within 2 weeks of vaccination included amiodarone, melatonin, venlafaxine hydrochloride (EFFEXOR), ibuprofen, aripiprazole (ABILIFY), lisinopril, cranberry capsules, diltiazem, paracetamol (TYLENOL), famotidine, furosemide (LASIX [FUROSEMIDE]), ipratropium bromide, salbutamol sulfate (IPRATROPIUM/ALBUTEROL), buspirone, senna alexandrina leaf (SENNA [SENNA ALEXANDRINA LEAF]), polyethylene glycol 3350 and morphine. The patient did not receive any other vaccines within 4 weeks prior to the COVID vaccine. Patient used took Penicillin, propranolol, quetiapine, topiramate, Lamictal and had allergy to them. Patient used took the first dose of BNT162B2 (lot number: EJ1685) via intramuscular at right arm on 21Dec2020 12:00 PM at single dose for COVID-19 immunization. Since the vaccination, the patient been tested for COVID-19 (Sars-cov-2 PCR) via nasal swab on 06Jan2021, covid test result was negative. Patient was found pulseless and breathless 20 minutes following the vaccine administration (11Jan2021 12:30 AM). MD found no signs of anaphylaxis. Patient died on 11Jan2021 12:30 AM because of cardiac arrest. No treatment received for the events. Outcome of pulseless and breathless was unknown. the autopsy was performed, and autopsy remarks was unknown. Autopsy-determined cause of death was unknown. It was reported as non-serious, not results in death, Life threatening, caused/prolonged hospitalization, disabling/Incapacitating nor congenital anomaly/birth defect.; Sender’s Comments: Based on the available information this patient had multiple underlying medical conditions including morbid obesity, diastolic CHF, epilepsy, pulmonary hypertension and COVID-19 diagnosed prior to vaccination. All these conditions more likely contributed to patients cardiac arrest resulting in death. However, based on a close temporal association (“”Patient was found pulseless and breathless 20 minutes following the second dose of BNT162B2 vaccine administration, contributory role of BNT162B2 vaccine to the onset of reported events cannot be completely excluded. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate.; Reported Cause(s) of Death: Cardiac arrest; Autopsy-determined Cause(s) of Death: autopsy remarks was unknown. Autopsy-determined cause of death was unknown” | |
| COVID19 VACCINE (COVID19) | Patient admitted for a fib; This is a spontaneous report from a contactable consumer. A female patient in her 70s received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE, Solution for injection) lot number was unknown, via an unspecified route of administration on 05Jan2021 at a single dose for COVID-19 immunization. The patient’s medical history and concomitant medications were not reported. It was reported that the patient received vaccine on Tuesday, 05Jan2021. On Thursday, 07Jan2021, patient did not feel good-pulse and blood pressure. It was mentioned that the patient admitted for a fib. Two days after receiving Pfizer-BioNTech Covid 19 vaccine, the woman patient in her 70s was admitted for a fib. Information about lot/batch number has been requested. | |
| COVID19 VACCINE (COVID19) | Vaccine administered at 08:16–08:25 patient c/o feeling unwell and dizzy 87, 143/84, 18, 100%; 08:28: Pt c/o dizzy & chest tightness, appeared pale, monitoring; 08:34: Pt c/o increased chest tightness 130, 144/81, 22, 100% monitoring 08:36: Epinephrine .3mg given IM to R thigh, pt. reported flushing, pounding heart, 911 called; 08:40: Pt flushed. | |
| COVID19 VACCINE (COVID19) | 5 days after Moderna vaccine, developed severe abd pain, mid epigastrium. No Nausea or vomiting. No fever. Mild diarrhea. after 48 hrs with no improvement went to ED | |
| COVID19 VACCINE (COVID19) | At first I has some injection site pain and soreness nothing too bad. But around 01:30 I awoke with a really high fever. My fever was 102.8 when I first woke up. I was very nauseous and my fever felt worse. My thermometer would not read any more until my temp came down. I can only guess how high it got but at least 103 degrees. I took Advil Liquid Gells and then my fever broke. I was actually scare for my life. In March I actually caught coronavirus and developed anti bodies for Covid. I can only guess my body was fighting for it’s life. | |
| COVID19 VACCINE (COVID19) | Actual event and cause of death were unknown; This is a spontaneous report from a non-contactable consumer. A 90-year-old female patient received first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration on 06Jan2021 at single dose for COVID Prevention. The relevant medical history included aortic valve replacement from Nov2019. Concomitant medications were not reported. The consumer stated that she was taking the reporting responsibilities to report that a friend of hers, informed that the patient passed away on Friday, and had received the COVID vaccine on Wednesday. The consumer stated that it was unknown to her at this time, if the friend had called to complete a report herself, regarding the incident. Their conversation was very brief. The patient was 90 years old, and it was her friend’s mother that was the patient. Actual event and cause of death were unknown. The patient had her vaccine on Wednesday 06Jan2021, and then the patient collapsed in front of the reporter at Friday night on 08Jan2021 and passed away that same day. The autopsy was unknown. The outcome of the event was fatal. No follow-up attempts are possible; information about lot/batch number cannot be obtained.; Reported Cause(s) of Death: Actual event and cause of death were unknown | |
| COVID19 VACCINE (COVID19) | My right leg from the knee down was purple and they thought I have a blood clot; My right leg from the knee down was purple and they thought I have a blood clot; This is a spontaneous report from a non-contactable consumer (patient). A patient of unspecified age and gender received bnt162b2 (PFIZER-BIONTECH COVID-19 mRNA VACCINE), via an unspecified route of administration on an unspecified date at single dose for COVID-19 immunization. The patient medical history and concomitant medications were not reported. Three days after vaccination the patient felt very sick and he/she was so bad that he/she thought he/she might die with outcome of recovered after one week. The patient reported also that on unknown date his/her right leg from the knee down was purple and they thought he/she have a blood clot, due to which the patient was hospitalized for 14 days. The patient ended in the hospital because his/her right leg from the knee down was purple and they thought he/she have a blood clot but they did an ultrasound that was not the case but they put he/she on antibiotic. The patient was still taking them but his/her leg has got better. No follow-up attempts are possible. Information about lot/batch number cannot be obtained. No further information is expected. | |
| COVID19 VACCINE (COVID19) | Covid symptoms began 2-3 days after shot. Presented to ER day 11 day after positive with extreme dyspnea O2 Sat at 88% Chest xray opacities throughout the bilateral hemithoracies would suggest multifocal infiltrate Admitted on high flow oxygen reduced to 6 liters currently D-dimer is >35.20 | |
| COVID19 VACCINE (COVID19) | Patient is a 99yr old female who got a covid vaccine in the afternoon of 1/10/21 and woke up in the morning of 1/11/21 with altered mental status, weakness, and dysarthria. She was taken from her assisted living facility to the hospital and MRI showed a small stroke in the right medial thalamus. She was also found to have new onset atrial fibrillation. She was treated appropriately for both conditions and discharged to a skilled nursing facility on 1/13/21. | |
| COVID19 VACCINE (COVID19) | Pt was accompanied by daughter, who drove pt to clinic. Pt was correctly identified, screened, given vaccine, monitored for appropriate time. Pt left clinic as passenger of vehicle and was being driven by daughter. Pt started complainingg of Shortness of breath and not feeling right.. Daughter brought pt to local emergency room, Pt was later transferred to higher care hospital. Information regarding symptoms and further medical care was relayed to clinic by family member. | |
| COVID19 VACCINE (COVID19) | Patient received vaccine in afternoon of 12/28. She works in ER as housekeeper 7pm-7am. The day she received the vaccine she became ill with fever chills and nausea and left work at 2am. On 12/31 she developed hemianopia. She went to ER and they did CT scan. She was told it was complex migraine. She left and came Home. On 1/1/21 her vision was back to normal. On 1/3 she suffered bilateral cerebellum ischemic stroke. She is currently in medical center. In Trauma. | |
| COVID19 VACCINE (COVID19) | Patient experienced a syncopal episode post vaccination, accompanied by feeling hot and tachycardic. Prior to the syncope, she reported hyperventilating. She remained unresponsive for about 10 minutes and was brought to the ED. There she became responsive, and reported chest pain and had sinus tachycardia episodes. She also had lower extremity weakness. This has improved per the latest neurology notes. She also had a negative EEG. Unsure if this is an allergic reaction or if the syncope was due to hyperventilation. Patient is currently on day 3 of hospitalization. | |
| COVID19 VACCINE (COVID19) | Systemic: Headache, Systemic: Eyes dilated and difficulty remembering information; symptoms lasted 2 days | |
| COVID19 VACCINE (COVID19) | I was short of breath and went to emergency room on 1/5/2021. I was diagnosed with bilateral pulmonary embolisms. I was Covid negative and had no other symptoms. | |
| COVID19 VACCINE (COVID19) | 2 Hours after the injection, my arm hurt so bad I could not raise it laterally. This continued for 3 days. After that I felt tired and achy until Jan 4th, I had chills and whole body aches. I came home from work, took the next day off. Feeling better, I worked 3 more days and developed the worst headache of my life. I consulted my PCP and went to the emergency room. I was diagnosed with viral meningitis and admitted to the hospital for 3 days. . | |
| COVID19 VACCINE (COVID19) | Staff walked into resident’s room around 10:00am and noted resident’s left side of his face was flaccid. Nurse was called and upon assessment resident noted to have an unequal hand grasp with left worse. He was able to talk but was mumbled and hard to understand. Physician, hospice, and family were notified. Resident had a stroke at 10:06 am on 1/8/2020. He lost all ability to use his left side. Resident passed away on 1/11/2020. | |
| COVID19 VACCINE (COVID19) | Patient received COVID-19 Vaccine at 0956 and reported symptoms of itchy face and chest pressure at approximately 1008 during observation period. Pt vital signs were 133/86, HR 130 and oxygen saturation 100% on room air. Pt reported worsening symptoms of chest pressure and itchiness to face. Provider instructed Epi Pen be given and pt to be transported to ED for further evaluation. EKG obtained and showed sinus tachycardia. Nonrebreather oxygen mask applied with 2L/min and oxygen saturation remained at 100%. Pt was transported via ambulance to at 1038 and pt reported feeling improved symptoms prior to leaving the clinic at approximately 1034. Pt stable at time of transfer. | |
| COVID19 VACCINE (COVID19) | Employee was awaken at 5:30 am on 1/13/2021 by chills and a feverish feeling. She then became nauseous and faint. She passed out and was noted by her mother who is a RN to have a seizure. She remained out for several minutes and then aroused. She has remained groggy the rest of today but has improved. She has a history of non-epileptic seizures since she was 14 and has been on medications for this. Employee stated she has not has any seizure activity in over a year. She did not see medical attention due to recovering quickly from this. | |
| COVID19 VACCINE (COVID19) | The patient passed away today, 1/13/2021. She was a hospice patient. She showed no adverse effects after receiving the vaccine on 1/12/2021. This morning she woke up as normal and during her morning shower she had a bowel movement, went limp and was non-responsive. The patient passed away at 7:45 am. | |
| COVID19 VACCINE (COVID19) | The morning following COVID-19 vaccination, patient’s right shoulder had swelling, generalized weakness and myalgia. Hospitalized for 2 days, received intravenous fluids and bedrest, and acetaminophen. He was prostrate for 2 days. | |
| COVID19 VACCINE (COVID19) | numbness to forearm then to lower leg that then took on a dermatomal pattern, brain fog w word finding issues that progressively worsened, LLE weakness. ct brain neg. MRI/MRA head and neck neg. labs neg. MRI of c spine t spine l spine s spine neg. emg and eeg neg. discharged from hospital. symptoms fluctuating. slowly improving. | |
| COVID19 VACCINE (COVID19) | Presented to MD’S office on 01/05/2021 with cough, HA, fever 101.9, chills, and fatigue. Returned on 01/07/2021 with no improvement. Returned on 01/11/2021 with fatigue, elevated temp 101.2, SOB, 02 Sat at 90%, Rocephin 1G IM administered, CXR revealed pneumonia. MD received call from patients husband stating worsening 02 Sat levels at 81-82%. Was transferred to hospital and admitted with pneumonia.. | |
| COVID19 VACCINE (COVID19) | This person was found to be deceased on routine rounds during the night, 3am. No symptoms of reaction noted post vaccine. No injection site reaction. No reports of any allergic reaction. | |
| COVID19 VACCINE (COVID19) | Resident began having fever on 1/11/21 @0600. VS= T-102 B/P- 100/57 P- 112 RR- 24 O2 Sat 92% on RA. MD called. Rapid COVID Test was negative. CBC,CMP, U/A were ordered as well as CXR. Resident’s condition declined. At 3:00pm resident started having respiratory distress and hypoxia O2 Sat 89%. Supplemental O2/mask @ 5LPM. Neb TX, EKG, and Rocephin 1 GM ordered. Condition worsened. Resident sent to nearest ER for evaluation. Later in the evening the staff AT Medical Center called to inform staff that resident had expired @ 2230 as a result of Respiratory Failure and Sepsis. | |
| COVID19 VACCINE (COVID19) | about 14 hours after vaccination I experienced what appeared to be a severe case of Cytokine storm. I had a moderate case of COVID in May 2020 and had positive IgG AB in August. The symptoms started with heavy shaking chills, lasting 1 1/2 hours , fever and most concerning sustained tachycardia with heart rate of 180′ to 200′ over hours, which then destabilized into runs of Vtach and complex ventricular dysrythmia, low BP, profound weaklness, head aches and joint and muscle pains ( similar to the experienced COVID symptoms ) | |
| COVID19 VACCINE (COVID19) | “Patient received vaccine at 0939. 30 minute wait period related to history of previous anaphylactic reaction. 10:05 patient walked from observation area to vaccinator and reported that her “”chest was burning”” and “”feels like it is getting tighter to breathe. Pt had change in voice. Patient moved to chair and started on oxygen 1.5 L/m per nasal cannula. MD came to room to assess patient. ED arrived and checked patient B/P. Patient transferred by cart to ED.” | |
| COVID19 VACCINE (COVID19) | Patient began having cramping of her upper extremities and subsequent swelling of her face and neck and also shortness of breath, chest pressure and flushed appearance. She denies any tongue or throat swelling. She is also complaining of bilateral arm pain. The initial reaction treatment was started in the conference room and then the patient was transferred to the emergency room. She continued to has arm and chest pain in the emergency room. She had muscle tension/rigidity in the upper extremity which caused significant pain. She also developed a nonspecific rash on the chest and abdomen that persisted for through out he hospital stay. Treatment was started with epinephrine 0.5 mg IM x2 and diphenhydramine 50 mg IM x1, prior to transfer to emergency room. In the emergency room treatment was dexamethasone 10mg IV x 1, famotidine 20mg IV x1, Ketorolac 30mg IV x 1, lorazepam, 1mg IV x 2, morphine 4mg IV x 2, 1000ml Saline Solution. As an inpatient the treatment included scheduled acetaminophen 500mg TID, as need morphine for pain after treatment with fentanyl, scheduled diphenhydramine 25mg IV q8 and compazine 5mg IV for nausea, cyclobenzapine 10mg as need for muscle spasms, dexamethasone as a scheduled dose started at 4 mg BID and tapered to 2 mg BID, Naproxen 500mg BID and Norco 7.5/325 as need for pain. with famotidine 20mg BID . | |
| COVID19 VACCINE (COVID19) | Note: I am currently breastfeeding. Had body aches; chills; fever of 102.6; headache; nausea; cough; shortness of breath – I went to ER and that is where I received COVID positive test and positive tests for COVID Pneumonia and Microplasm Pneumonia as well. Stayed overnight in ER observation – 2 am to 8 am . Went home next morning and was quarantined approximately 8 days. Body aches and a cough had started two days prior to injection. IV antibiotics at the hospital and oral antibiotics at home. Received nebulizer breathing treatments. Pain meds and anti-inflammatory medication. | |
| COVID19 VACCINE (COVID19) | Onset of tachycardia was 8:30pm on 1/12/21 with a noted HR of 164 and SR. Went to ER and had HR of 171 upon arriving to triage window. EKG said Sinus Tachycardia. Admitted to observation station with telemetry to monitor HR. HR normalized around 100 when up and walking at 10:45am on 1/13/21. | |
| COVID19 VACCINE (COVID19) | I received my covid vaccine on 12/29/2020 at 4:42pm. In 10 minutes after the shot, I went into anaphylactic shock, I broke out in hives, and my throat was closing | |
| COVID19 VACCINE (COVID19) | Pt reported chest tightness and throat tightness following vaccination. Time course following vaccination unknown. |
| COVID19 VACCINE (COVID19) | little bit of a reaction light headed after 5 minutes. vitals were low, so observed for 30 minutes after being light headed. Patient was found unresponsive and pronounced dead later that day. | |
|---|---|---|
| COVID19 VACCINE (COVID19) | Death occurred 3 days after vaccine receipt; attributed to complications of her chronic advanced dementia with aspiration at age 87. No evidence of acute vaccine reaction. | |
| COVID19 VACCINE (COVID19) | No adverse effects from vaccination seen on 1/2/21. On 1/6/21 resident was seen by Dr and her baclofen pump was refilled with 20 ml Baclofen 4,000mcg/ml. ITB Rate increased by 6% to 455.5 mcg/day simple continuous rate over 3 days. On 1/8/21 at 0615 resident was shaking, lower extremities mottled, Sa02 70%, pulse 45. Oxygen started at 2 L/m per NC. At 0715 her primary physician was notified as well as her daughter. Oxygen increased to 4 L/min, sats at 83%. SOA noted, reported all over pain. At 0850 when they attempted to reposition the resident, she was not responsive. Licensed nurse assessed her and no heartbeat heard or pulse found. | |
| COVID19 VACCINE (COVID19) | I had a mild headache the evening of the shot, I had a headache the next two days that was relieved by Advil. I had a very sore arm at the injection site Friday and Saturday after the shot was given. The arm pain was gone Sunday morning. I was very tired on Saturday especially and slept through the morning and early afternoon on and off until about 3:00 pm. On Friday during the day, I noticed my right ear starting to feel unusual and uncomfortable. On Saturday, the ear issue continued, I felt like I had some hearing loss and a constant buzzing and ear fullness feeling. On Sunday, the ear issued continued with the hearing loss, buzzing and fullness and has through today and hasn’t stopped. I tried some nasal decongestant on Sunday afternoon, but it didn’t have any effect. I made an appointment with the ENT doctor on Monday morning for Tuesday. I had a hearing test on Tuesday and saw the ENT doctor on Wednesday. He prescribed prednisone and ordered an MRI. I will be starting the prednisone later today (Wednesday) when the pharmacy has the prescription ready. | |
| COVID19 VACCINE (COVID19) | 54 y/o M with PMH of HTN, HLD, Alcoholic Cirrhosis, Aortic Valve Stenosis, and angina BIBA as a Medical Alert for cardiac arrest noted PTA. Per EMS, the patient called because he was having constant, diffuse abdominal pain x 1 day that radiated to his chest. On scene, the patient had a witnessed arrest with EMS starting CPR. He was given 3 rounds of epi without ROSC. Pt had no associated shockable rhythm. Of note, pt’s wife, had noted pt had received covid vaccine the prior day. | |
| COVID19 VACCINE (COVID19) | A few minutes after the vaccine, she had mild tongue swelling. It only lasted a few minutes, and then she felt fine. However, her BP went up. I’m not sure how high. she then has a short run of asymptomatic V tach. Taken to hospital observed overnight. No further events or problems. | |
| COVID19 VACCINE (COVID19) | She got the vaccine on Dec 23, and then on Jan 4 she had a mild stroke with left sided arm and face weakness. She did recover fully. She already has known CAD and risk factors for CVD. It is possible, but by no means certain, that the vaccine was an indirect cause of the event. Since the vaccine provoked an immune response, as it was supposed to, it is possible that this inflammation may have set up a metabolic predisposition that may have contributed to the event, which was 12 days later. | |
| COVID19 VACCINE (COVID19) | New onset altered mental status after fall. Per son, she was found in bed, unresponsive at around 12:15 AM on 1/10 by her husband. Brought to the emergency room and admitted, Began treatment for UTI w/ CTX. Discharged 1/11/21 with f/u appointments | |
| COVID19 VACCINE (COVID19) | Resident received 1st dose on 1/4/2021. On 1/6/2021 resident having SOB, increased weakness with O2 sats at 91% RA. On 8th resident sustained a fall, O2 sats 88-92, dizzy, weakness. Rapid COVID test performed with negative results. Evening of 8th resident was lethargic and diaphoretic with fever of 99.9. Resident transferred to ER, on 5lt of oxygen. Resident returned from the ER on 1/9/2021 with new diagnosis of Leukemia and orders for hospice. Continued with fever, crackles and N/V and loss of appetite from the 9th and 10th of January. Resident expired at 820am on 1/11/2021. | |
| COVID19 VACCINE (COVID19) | I had a headache at the top of my head, and a tingling sensation down my left leg from my buttocks to my calf. The tingling lasted until the next morning then went away. On 1/3/2021 I experienced a strong headache followed by facial numbness and tingling. This lasted for an hour or so and slowly subsided. The headache lingered for 3 days. | |
| COVID19 VACCINE (COVID19) | Headache; migraine; tenderness at injection site; Ten hours after injection he had shaking, sweats, hot and cold flashes, and augmentation of myalgias; Ten hours after injection he had shaking, sweats, hot and cold flashes, and augmentation of myalgias; Ten hours after injection he had shaking, sweats, hot and cold flashes, and augmentation of myalgias; Ten hours after injection he had shaking, sweats, hot and cold flashes, and augmentation of myalgias; Ten hours after injection he had shaking, sweats, hot and cold flashes, and augmentation of myalgias; Fatigue; This is a spontaneous report from a contactable physician (patient). A 53-year-old male patient received second dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE, lot number: EK9231), via an unspecified route of administration on right deltoid on 05Jan2021 07:45 at single dose for covid-19 immunization. Family history included migraine (other family members). Medical history included mild blood pressure and kidney stones, reactive airway disease. Concomitant medication included colecalciferol (VITAMIN D), potassium, allopurinol and hydrochlorothiazide/valsartan for mild blood pressure and kidney stones, fluticasone propionate, salmeterol xinafoate (ADVAIR) for reactive airway disease, atorvastatin, and multivitamins. The patient previously took fluticasone propionate, salmeterol xinafoate (ADVAIR) and experienced dry mouth and lost sense of taste. The patient also previously took Tdap booster on Aug2020, Shingrix on 10Aug2020, and influenza on 12Oct2020; all for immunization; and tetanus injections for immunization and experienced localized tenderness. The patient had the first dose of BNT162B2 (lot number: EH9899) for COVID-19 immunization on 15Dec2020 and experienced localized tenderness at injection point. The received his second dose of COVID vaccine on 05Jan2021. With the first dose he had increased localized tenderness at injection site on 15Dec2020, and he rated it mild to moderate. He would say it was 80% resolved in 24 hours. It had completely resolved in 36 hours. He would say that he has recovered completely form the localized tenderness with the first dose. Then he noted his second dose was yesterday, in the context of not having much sleep the night before. The actual injection was uncommonly eerily painless. The other folks in his department had similar experience. Maybe it was the nurse who gave the injection. Maybe it was because it was the same area and sensitivity was decreased. They had to check the Band-Aid to make sure blood was there. The administration was painless. He was relieved when the arm started getting sore to know he actually received it. He had increased arm tenderness at injection site which he rated as moderate which has now resolved. It got to moderate where lifting the arm up was sore. He definitely knew that he had been vaccinated. He got the vaccine at 7:45AM and now it is 16 to 17 hours later and he would say the pain is mild now. It did persist. The first vaccine hurt a little more. He expects this to go away. Ten hours after injection he had shaking, sweats, hot and cold flashes, and augmentation of myalgias. He had unrelenting headache over night that was moderate to severe. He said it kept him awake. It was exacerbated by lying down. Sitting up helped him. It became a migraine which is something he doesn’t often experience. Migraines are pretty rare for him. He took 800mg of Advil at 6AM that helped for headache and migraine. The weight of the patient was 250 to 255 pounds. Shaking, sweats, hot and cold flashes, and augmentation of myalgias have resolved. Everything has resolved except for a little headache. In the background he literally had one or two hours of sleep. He thinks that likely precipitated a migraine was increased. Last night he slept literally an hour. He took 800mg of Advil and fell asleep. He is operating on 2 hours of sleep in 48 hours. Most of the stuff is gone except a little headache and expected fatigue. Headache Seriousness Criteria: he would say that it was relatively disabling. He would not have been able to carry on. He wouldn’t have been able to operate last night. It would have interfered. It was dissimilar to others. He gets rare migraines. Everything was amplified with a migraine. He certainly felt that. It was fair to say the vaccine precipitated the migraine that was mild or severe. He doesn’t want to falsely attribute these things to the vaccine. Causality Headache: precipitated by the vaccine. In the context that he had not slept the night before. He had a nasopharyngeal COVID test and it was negative. He has been in a COVID study where they are looking at combination. They developed a saliva test at (Name). There is a combination of saliva oropharyngeal and immunoglobins. He has been negative multiple times. The outcome of the events headache and fatigue was not recovered and recovered for the rest of the events.; Sender’s Comments: A causal association between BNT162B2 and the reported event headache cannot be excluded based on a compatible temporal relation between vaccination and onset of events. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to RAs, Ethics Committees, and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | severe angioedema, hives, tachycardia, hypertension, pruritus, chest tightness and shortness of breath.; severe angioedema, hives, tachycardia, hypertension, pruritus, chest tightness and shortness of breath.; severe angioedema, hives, tachycardia, hypertension, pruritus, chest tightness and shortness of breath.; severe angioedema, hives, tachycardia, hypertension, pruritus, chest tightness and shortness of breath.; severe angioedema, hives, tachycardia, hypertension, pruritus, chest tightness and shortness of breath.; severe angioedema, hives, tachycardia, hypertension, pruritus, chest tightness and shortness of breath; severe angioedema, hives, tachycardia, hypertension, pruritus, chest tightness and shortness of breath; This is a spontaneous report from a contactable nurse (reporting for herself). A 41-year-old non-pregnant female patient received two doses of BNT162B2 (PFIZER-BIONTECH COVID-19 MRNA VACCINE), both via an unspecified route of administration in the left arm, the first dose on 16Dec2020 09:00 (lot number: EH9899) and the second dose on 08Jan2021 07:15 (lot number: EL0140), both at a single dose for COVID-19 immunization. Medical history included ongoing anxiety, from an unspecified date. The patient had no known allergies. Concomitant medication included escitalopram oxalate (LEXAPRO), acetaminophen (MANUFACTURER UNKNOWN), naproxen sodium (MANUFACTURER UNKNOWN), ibuprofen (MANUFACTURER UNKNOWN). The patient did not receive any other vaccines within 4 weeks prior to the COVID vaccine. Prior to vaccination, the patient was not diagnosed with COVID-19 and since the vaccination, has not been tested for COVID-19. On 09Jan2021 at 01:30 AM, the patient experienced severe angioedema, hives, tachycardia, hypertension, pruritus, chest tightness and shortness of breath, all of which were reported as being life-threatening. The patient went to the Emergency room due to the events. Therapeutic measures were taken as a result of the events and included: methylprednisolone sodium succinate (SOLUMEDROL) 125 mg, famotidine (MANUFACTURER UNKNOWN) 20 mg and diphenhydramine hydrochloride (BENADRYL) 50 mg. The clinical outcome of severe angioedema, hives, tachycardia, hypertension, pruritus, chest tightness and shortness of breath was recovering.; Sender’s Comments: A possible causal association between administration of BNT162B2 and the onset severe angioedema, hives, tachycardia, hypertension, pruritus, chest tightness and shortness of breath cannot be excluded, considering the plausible temporal relationship and the known adverse event profile of the suspect product. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | Received 2nd vaccination in series on 11 JAN 21. By next morning started to experience some muscle and joint symptoms. By afternoon of 12 JAN 21 experienced sudden onset loss of bladder control for first time in his life, followed a few hours later by blurry vision, vertigo, motion sickness, emesis and cold sweats that drenched his clothes. Taken to ER by paramedics, and admitted for further observation and evaluation for underlying neurologic vs. cardiogenic problems. Given positional treatments by PT for possible otoliths. Feels much improved on afternoon of 13 JAN 21, but symptoms not fully resolved. Will likely be discharged from hospital on 14 JAN 21 if problems continue to resolve. | |
| COVID19 VACCINE (COVID19) | Admission Note: ? Weakness – Generalized á á Patient reports feeling weak prior to dialysis, but demanded clinic to perform dialysis. Had full tx done and brought to ER. Reports still feels weak after dialysis. á 84 year old male comes in today after completing dialysis for evaluation of generalized weakness x 5 days. He has also lost his voice. He tells me he received his COVID vaccine yesterday, but he is concerned he may have COVID. He denies any fevers, cough, sore throat, NVD, abd pain. Transfer Note: HOSPITAL COURSE: Patient is a 84 y.o. male who presented with shaking chills and was found to have Gram-negative rods in the blood. The source of infection was unclear. Initially it was thought that it could possibly be cholecystitis but imaging was negative for that. There was concern that it could be UTI but the patient is on dialysis and is an uric and therefore no urinalysis could be got. Early this morning when I saw the patient the patient did have significant pain and tenderness in the right knee and is not able to put weight on that. I.e. Consulted Dr. Today with per lumbar from Orthopedics who said that it would be in the best interest of the patient for him to be transferred to hospital where he could decide on aspiration and or washout of the right knee. á Transfer center has been called and we are trying to finalize a transfer of the patient hospital at this point of time á Please see problem list listed below. á REASON FOR ADMISSION/ ADMISSION DIAGNOSES á Sepsis cause unclear | |
| COVID19 VACCINE (COVID19) | History of Present Illness: á Patient is a 80 y.o. male who presents with chest pain. Patient reported that he 1st had the chest pain approximately 2 weeks ago when he woke from sleep. At that time patient pain lasted about 5 minutes or so and resolved when he got out of bed. He did well for the rest of the day up until yesterday. Patient reported that yesterday morning he woke up with the pain at the lasted about 30 minutes or so. Patient also had associated burping felt that it is likely GI in nature. The pain was located mainly in the left side of the chest without any radiation. No diaphoresis. No shortness of breath or palpitation. No radiation for the pain. Since yesterday morning he had another 3 episodes of pain the last after dinner tonight. Patient reported that this pain was located more on the left side of the chest, likely lasted about 10 minutes or so. There was no exertional component to the pain. No known history of heart disease. Due to rather recurrent nature of the pain patient was brought to the hospital by his son who is a cardiologist to be evaluated. No fever or chills. No cough . Patient reported that he got vaccination for COVID 2 days ago-of a concern that this may be a side effect of the vaccine. No dizziness lightheadedness. Patient with history of GI bleed in the past at that time patient was on NSAIDs. Patient with burping associated with the pain | |
| COVID19 VACCINE (COVID19) | Chief Complaint Patient presents with ? Generalized Body Aches á á Pt presents via EMS c/o DOE, dry non-productive cough, subjective fevers Tmax 101.9, decreased appetite, aches since testing + for COVID on 1/5. á Patient is a 50 year old male with PMH of Crohns/MS on fingolimod presenting to the Hospital for fevers, shortness of breath and weakness. Patient received COVID vaccine on 12/29. Patient had initial left arm discomfort though has had worsening weakness, cough, shortness of breath and fevers since that time. Patient tested positive for COVID19 on Patient has shortness of breath with exertion that is relieved by rest. Patient denies N/V/D. Patient has taken tylenol at home to attempt to alleviate symptoms. | |
| COVID19 VACCINE (COVID19) | Chief Complaint Patient presents with ? Vomiting á á pt reports dry heaving and nausea that started an hour ago á Patient is an 68 y.o. year old male with PMHx significant for HTN, BPH, who presents to the ED today with nausea and dry heaving for one hour PTA. States that he was at work as a courier when he had onset of sensation of room spinning, nausea, and dry heaving. Also having tinnitus which he thinks is b/l. This happened once about two weeks ago and resolved spontaneously overnight when he was asleep. No preceding illnesses, medication changes, or other associated symptoms. Vertigo has no clear exacerbating or relieving factors. Has not yet taken anything for symptoms. á The patient denies fevers, chills, headaches, syncope, chest pain, shortness of breath, rhinorrhea, sore throat, cough, abdominal pain, changes in usual bowel movements, changes with urination, back pain, pain anywhere else in body. The patient has no sick contacts, recent travel history. | |
| COVID19 VACCINE (COVID19) | HOSPITAL COURSE: Patient is a 50 y.o. female with a history of anxiety and migraine headaches who presented to hospital with cough and diarrhea. The patient had felt the symptoms on 12/31/2020 and eventually went to have a COVID test on 1/1/2021. She was subsequently positive but due to dehydration and diarrhea she came to the emergency department where she was admitted for remdesivir and dexamethasone. She was able to be weaned off any supplemental oxygen. Her diarrhea resolved. She is feeling well and will be discharged home in good condition. | |
| COVID19 VACCINE (COVID19) | None stated. | |
| COVID19 VACCINE (COVID19) | Peripheral neuropathy | |
| COVID19 VACCINE (COVID19) | Pt experienced 103.7 fever and muscle spasms. Hospitalized. | |
| COVID19 VACCINE (COVID19) | Numbness tingling in feet, toes progressed to waist. ER Sunday hosp, pt was admitted inpatient, diagnosis transverse myelitis. | |
| COVID19 VACCINE (COVID19) | Systemic: Dizzy, hyperventolation-Medium | |
| COVID19 VACCINE (COVID19) | Began with tingling/itching to tongue and roof of mouth approx 15 minutes after administration, progressed to tingling of lips, was sent to the ED for observation. Within 20-30 minutes developed cough, throat tightness, difficulty swallowing, breathing, vomiting, shortness of breath. Noted to have uvular swelling and wheezing on examination. Given Benadryl, Pepcid, Solumedrol, Zofran, Albuterol MDI, Epi IM. within a few minutes symptoms returned and were worse where I felt like I could not breathe, throat was closing, could not talk. Noted to be pale, HR in 140?s. Given second dose of epi IM and symptoms improved. Was transferred to Obs Unit., within 2 hours (approx 6 hours after administration), developed SOB, throat tightness, cough, vomiting, difficulty breathing. Again noted to have swelling of uvula, wheezing on exam. Given Solumedrol, Benadryl, SQ epi, Albuterol, Racemic Epi nebulizer. Was transferred to ICU, all meds held except Pepcid. Day #2 ~10 am (25 hours from administration) developed throat tightness, diffuse red rash to arms, difficulty breathing, vomiting. Again noted to have uvular swelling and wheezing. Given Solumedrol, Benadryl, Pepcid, Albuterol MDI, Racemic Epi neb. Solumedrol started q12hour dosing. Strange feeling/fullness in throat continued all day, got additional racemic Epi neb that night with improvement of symptoms. Following morning (day#2 after vaccine) noted to have diffuse red rash to chest and face, spread to arms, then began coughing. Given Solumedrol, Pepcid, Benadryl, Advair, Racemic Epi nebulizer. Solumedrol changed to q8 dosing. Approx 4 hrs later nurse noted rash worse on face, associated with itching, throat tightness. Given additional Benadryl, Racemic Epi neb with improvement. Rash continued that night with throat tightness, got additional Benadryl and Racemic Neb that night (total of 3 Racemic nebulizer on Day#2 post vaccine). Transferred to telemetry floor. Day#3 post vaccine rash improved, but still present to chest and face. Throat fullness present, especially after drinking. Am still hospitalized while writing this report | |
| COVID19 VACCINE (COVID19) | Initial pain in back of head and extreme headache. Some vomiting. At emergency, went into coma and was intubated. Hole drilled in skull to relieve pressure. MRI taken. Lot of bleeding in brain – anuerism lead to death approximately 14 hours after initial symptoms. | |
| COVID19 VACCINE (COVID19) | Systemic: Anaphylaxis-Medium; symptoms lasted 1 day | |
| COVID19 VACCINE (COVID19) | Unprovoked seizure (clonic tonic) 13 days later, requiring hospitalization and testing | |
| COVID19 VACCINE (COVID19) | Pt collapsed at home approx 5:30 pm and died | |
| COVID19 VACCINE (COVID19) | On day due for 2nd dose, Patient was found unresponsive at work in the hospital. Patient pupils were fixed and dilated. Full ACLS was initiated for 55 minutes with multiple rounds of bicarb, calcium chloride, magnesium, and epinephrine. Patient was intubated. Patient continued into V. Fib arrest and was shocked multiple times. | |
| COVID19 VACCINE (COVID19) | Systemic: reported by staff patient expired under suspicious circumstnces after receiving vaccine. Patient was on hospice, reported not expected to pass this soon; symptoms lasted 0 days | |
| COVID19 VACCINE (COVID19) | Extreme lockjaw unable to barely talk or chew | |
| COVID19 VACCINE (COVID19) | Day 7 post vaccine, woke with vertigo, nausea and double vision prior to my alarm going off at 5:30am, told my husband that I did not feel right and it felt like something was wrong with me. I asked him to send a message to work that I was not feeling well and would not be in. I was not able to see my phone clearly as I had double vision. I went back to sleep to try to get relief, woke up and called out to husband for help and he said I was just making groaning noises but I know in my head I was saying please help me as I could not move my right side and I was not sleeping or having a dream. At approximately 9:30am my husband kept telling me someone was trying to text me and needed a response. I tried to get up and still could not move my right side. He helped me sit up and I could not use my right arm and hand. He helped me get some clothes on and took me to the ED. When we arrived I still could not use my right hand and my arm still felt weak. The weakness resolved within a couple of hours and I got the strength back in my hand. I did have some concern that my TIA type symptoms could possibly be related to vaccine but it was just a thought until I received my second dose. 3 hours post vaccine my face became numb and tingly. It remained that way until I went to sleep. This is why I am reporting this incident. | |
| COVID19 VACCINE (COVID19) | Per patient report on follow-up: admitted to hospital following initial vaccine on 12/29 with N/V and severe HA. Patient placed on Morphine for pain, now resolved. Admission occurred outside of hospital system providing initial vaccine. | |
| COVID19 VACCINE (COVID19) | Patient reports no symptoms until 1/8/21 at which time a rash developed along with fatigue and fevers. Patient was seen in ED 1/8 and 1/11/21. Was admitted 1/11/21 with Concern for STevens Johnson and sepsis. Patient subsequently developed full body macular rash and mucosal lesions. Fevers to 102-104. | |
| COVID19 VACCINE (COVID19) | Reported redness, swelling and pain at injection site, diarrhea and light headedness1/5/21. Evaluated in ED and hospitalized for neurological symptoms that started 1/8/21. | |
| COVID19 VACCINE (COVID19) | vaccinated at Hospital on 1/12/2021. According to patients father she developed stomach pains, constant vomiting, sweating which started at approx. 4am. She arrived at Medical center at approx. 7:20 stating she had been vomiting since 4am. Attempted PO fluids while in ED. Uncontrolled nausea persisted despite medication. Admitted to Hospital for observation for uncontrolled nausea. IV fluids given | |
| COVID19 VACCINE (COVID19) | Developed pulmonary embolism in right lung one week after vaccination. Sharp pain on right side when breathing. Treated with IV Apixaban while inpatient for 2 days, oral Apixaban 5 mg, 2 tabs twice daily 1/5/21-1/11/21, then one 5 mg tab twice a day. Pain has subsided as of 1/14/21. | |
| COVID19 VACCINE (COVID19) | No adverse reactions observed after administration of medication. Patient starting complaining of shortness of breath around 0500 the following morning. SP02 checked in the 80s. Patient expired 01/09/2021; | |
| COVID19 VACCINE (COVID19) | Sudden death; This is a spontaneous report from a contactable physician from the regulatory authority. The regulatory authority report number is GB-MHRA-ADR 24556755. An 86-year-old male patient received BNT162B2 (PFIZER-BIONTECH COVID-19 mRNA VACCINE; Lot number EJ0553), via an unspecified route of administration on 19Dec2020 as single dose for COVID-19 immunization. Medical history included Waldenstrom’s macroglobulinemia for Waldenstrom’s macroglobulinaemia, memory impairment, with recent memory problems, cerebrovascular accident , with previous CVA, joint swelling , reported as slight ankle swelling awaiting head CT scan and bloods, oesophagitis, and cholesterol, all from an unknown date and unknown if ongoing. Concomitant medication included acetylsalicylic acid (MANUFACTURER UNKNOWN) for Waldenstrom’s macroglobulinaemia, lansoprazol (MANUFACTURER UNKNOWN) for oesophagitis, simvastatin (MANUFACTURER UNKNOWN) for blood cholesterol. The patient had sudden death on 29Dec2020. The patient died on 29Dec2020. It was not reported if an autopsy was performed. The reporter did not think the COVID vaccination caused the patients death; It did not appear to be related. The patient was seen by the physician on the 24th (not otherwise specified), and was fine. The patients son also saw the patient on the 28th (not otherwise specified) and also fine with no side effects from the jab. No follow-up attempts are possible. No further information is expected.; Sender’s Comments: Based on the information currently provided, the company considers the patient death is unrelated to the vaccine use; the advance old patient having multiple pre-existing medical conditions including Waldenstrom’s macroglobulinaemia and cerebrovascular accident, which more likely led the patient to sudden death.; Reported Cause(s) of Death: Sudden death unexplained | |
| COVID19 VACCINE (COVID19) | SARS-CoV-2 infection; SARS-CoV-2 infection; This is a spontaneous report from a contactable physician received by Regulatory Agency . The regulatory authority report number is GB-MHRA-ADR 24558365 & GB-MHRA-WEBCOVID-20210105143744. A patient of unspecified age and gender received BNT162B2 (PFIZER-BIONTECH COVID-19 MRNA VACCINE), via an unspecified route of administration, on an unspecified date at a single dose for COVID-19 immunization. The patient’s medical history and concomitant medications were not reported. Patient was not enrolled in clinical trial. The patient experienced SARS-coV-2 infection on 27Dec2020. The patient underwent lab tests and procedures which included COVID-19 virus test: yes – positive covid-19 test on an unspecified date. The clinical outcome of SARS-coV-2 infection was fatal. The patient died on an unspecified date. An autopsy was not performed. No follow-up attempts are possible; information about batch/lot number cannot be obtained.; Reported Cause(s) of Death: SARS-CoV-2 infection | |
| COVID19 VACCINE (COVID19) | Cardiac arrest; This is a spontaneous report from a contactable physician. This is a report received from the MHRA. Regulatory authority report number GB-MHRA-WEBCOVID-20210105171610, Safety Report Unique Identifier GB-MHRA-ADR 24558665. A male patient of an unspecified age received BNT162B2 (Pfizer-Biontech Covid-19 Vaccine), via an unspecified route of administration on 23Dec2020, at single dose for covid-19 vaccination. Medical history included ongoing dementia, and cardiac pacemaker insertion on an unknown date. Patient has not had symptoms associated with COVID-19. Unsure if patient was enrolled in clinical trial. The patient’s concomitant medications were not reported. The patient experienced cardiac arrest on 31Dec2020. Had spontaneous cardiac arrest 9 days (to be clarified) after vaccination doubtful implicated but new vaccine of course. Patient had not tested positive for COVID-19 since having the vaccine. The patient underwent lab tests and procedures which included COVID-19 virus test: no – negative covid-19 test on an unspecified date. The patient died of cardiac arrest on 31Dec2020. It was not reported if an autopsy was performed. Information about lot/batch number cannot be obtained.; Reported Cause(s) of Death: Cardiac arrest | |
| COVID19 VACCINE (COVID19) | This is a spontaneous report from a contactable consumer. This consumer reported different fatal events for four patients. This is the second of four reports. An 82-year-old female patient in a nursing home received the first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE lot number: EK4238) via an unspecified route of administration on 04-Jan-2021 at a single dose for COVID-19 immunisation. Medical history included background of asthma, dementia, depression, gastrointestinal and heart failure. Concomitant medications were not reported. 4 Hours after the receipt of the vaccine, she was found in her room on the floor with a bruise on her forehead apparently from a fall, CPR was performed by nursing home staff. Staff performed CPR, asystole without heart sounds, CPR continued for 23 minutes without any change and death was declared. The events occurred in Jan 2021. The date of death was in Jan 2021. The outcome of events was fatal. It was unknown if an autopsy was performed. Sender’s Comments: Linked Report(s): IL-PFIZER INC-2021019507 Same reporter, same product, different patient/events; Reported Cause(s) of Death: was found in her room on the floor with a bruise on her forehead apparently from a fall; was found in her room on the floor with a bruise on her forehead apparently from a fall. | |
| COVID19 VACCINE (COVID19) | hemolytic anemia; reduced air entrance; passed away; low blood pressure; jaundice appeared on the whole body with lymphocytosis; jaundice appeared on the whole body with lymphocytosis; shortness of breath in mild efforts; weakness which expressed by shortness of breath in mild efforts; hands tremor; shortness of breath; This is a spontaneous report from a contactable consumer received via regulatory authority. This consumer reported different fatal events for four patients. This is the third of four reports. A 72-year-old male patient received the first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE; lot number was not specified) via an unspecified route of administration on 21Dec2020 at a single dose for COVID-19 immunisation. Medical history included Kerattis, prostatectomy other, blood pressure problems (treated with nifedipine and hydrochlorothiazide/ramipril (TRITACE COMP)), hyperlipidemia (treated with statins), oncological patient-underwent radical restriction of the prostate, and sensitivity to phenylephrin. Concomitant medications were not reported. Three days after the vaccine (on 24Dec2020) he started to feel shortness of breath, arrived for hospitalization 10 days after vaccination. Five days after vaccination (on 26Dec2020) he experienced weakness which expressed by shortness of breath in mild efforts, hands tremor. 6 days after vaccination (on 27Dec2020) jaundice appeared on the whole body with lymphocytosis. On the day after, he referred to the physician and blood tests were sent. He was hospitalized following diagnosis of hemolytic anemia. He received two blood doses and steroids. Two hours before he passed away, low blood pressure was measured and reduced air entrance, CPR was performed without success and the patient passed away. The date of death was unknown. The cause of death was unknown. It was unknow if an autopsy was performed. The outcome of event unknown cause of death was fatal, and of other events was unknown. Information on the lot/batch number has been requested.; Sender’s Comments: Linked Report(s) : IL-PFIZER INC-2021019507 Same reporter, same product, different patient/events; Reported Cause(s) of Death: passed away | |
| COVID19 VACCINE (COVID19) | altered mental status, hypoxic, fever 39.3, agitated | |
| COVID19 VACCINE (COVID19) | On 1/12/20 resident woke up and was not able to stand in the E-Z stand. E-Z lift was needed. In addition he needed assistance with eating. At that time VS were stable, equal hand grasp noted, and no further concerns. Around 3pm resident became flaccid on the left side of his face and speech became mumbled. Hand grasp was equal at that time and VS were stable, but B/P was elevated compared to previous recordings earlier in the day. Family did not want him sent to the hospital and asked for comfort cares. Hospice referral obtained and he will be admitted to hospice in the near future. Resident’s left side of face has improved within the last 48 hours. He remains total assist with all cares. | |
| COVID19 VACCINE (COVID19) | Myocardial Infarction; This is a spontaneous report from a contactable Other healthcare professional (patient). A 64-year-old male patient received first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE, Solution for injection, Batch/lot number: 20201216-1), via an unspecified route of administration on 16Dec2020 08:15 at single dose for COVID-19 immunization, vaccine location provided as Left arm. Medical history included arthritis and sulfa allergy. Prior to vaccination, the patient was not diagnosed with COVID-19. The patient’s concomitant medications were not reported. The patient did not receive any other vaccines within 4 weeks prior to the COVID vaccine. The patient experienced myocardial infarction on 19Dec2020 23:00. The patient was hospitalized for myocardial infarction for 3 days. The patient underwent lab test which included Covid test via Nasal Swab post vaccination on 20Dec2020 with test result Negative. Therapeutic measure Cardiac cath procedure was taken as a result of myocardial infarction. The outcome of the event was recovering. This case was reported as Serious with seriousness criteria hospitalization.; Sender’s Comments: The Company cannot completely exclude the possible causality between the reported myocardial infarction and the administration of COVID-19 vaccine, BNT162B2, based on the reasonable temporal association. However, more information is required, such as the complete medical history, clinical course, for the Company to make a more meaningful causality assessment. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to RA, IEC, as appropriate. | |
| COVID19 VACCINE (COVID19) | He collapsed with left sided hemiparesis; Stroke; Rt basal ganglia hemorrhage w/ edema and mass effect.; Rt basal ganglia hemorrhage w/ edema and mass effect.; Low platelets, 114; His bp as high as 200s/100; Hand weakness; Myalgia; Fever; Severe fatigue; This is a spontaneous report from a contactable physician. A 58-year-old male patient received first dose of bnt162b2 (Pfizer BioNTech COVID vaccine), intramuscularly on 16Dec2020 at a single dose for COVID-19 immunization. Medical history included hypertension with reported med noncompliance in the last few months due to stress. Concomitant medication included hypertension medications in two weeks. The patient was presumed neg covid status prior to vaccine. He worked as a Pulm/critical care physician. He reported fever, myalgia, fatigue on 16Dec2020. Next day (17Dec2020), he took off from work due to his symptoms. The following day (18Dec2020), he came to work. He c/o ongoing severe fatigue & hand weakness in am. Staff noted him to be evaluating his hands during clinic. At 12:15, he collapsed with left sided hemiparesis. The reporter had suspicion for stroke. He was transported to the Emergency Room (ER), head CT showed Rt basal ganglia hemorrhage w/ edema and mass effect. Labs notable for Low platelets, 114 (unknown baseline) on 18Dec2020, normal coags on an unspecified date. BP recorded as 179/101, but it was noted in trauma room his bp as high as 200s/100. He had a history of hypertension with reported med noncompliance in the last few months due to stress. Patient was transferred for further care. Full course was unknown but had rebleed there with low plts. Adverse event (he collapsed with left sided hemiparesis) resulted in hospitalization (22 days), life threatening illness (immediate risk of death from the event), disability/incapacitating or permanent damage. Treatment was received for adverse events. Results of tests and procedures for investigation of the patient: on 18Dec2020, Nasal Swab test: negative. The outcome of events was not recovered. Unknown if any other vaccines within 4 weeks prior to the COVID vaccine. Prior to vaccination, the patient was not diagnosed with COVID-19. Since the vaccination, the patient was not tested for COVID-19. Information on the lot/batch number has been requested.; Sender’s Comments: Collapsed with left sided hemiparesis/suspicion for stroke are as consequences of basal ganglia hemorrhage with edema, which is caused by worsening of hypertension. Low platelet also contributes to brain hemorrhage. All these serious events are unrelated to the vaccine use. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | “Heart rate shot up to 170 with numbness of feet.; Heart rate shot up to 170 with numbness of feet.; Chest pain; feeling like I was going to vomit and faint; feeling like I was going to vomit and faint; This is a spontaneous report from a contactable other hcp (patient). A 25-year-old non-pregnant female patient received the first dose bnt162b2 (PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration on 06Jan2021 at 11:45 at single dose in left arm for covid-19 immunization. There was no medical history. Concomitant medication included valaciclovir hydrochloride (VALTREX), topiramate (TOPAMAX), lamotrigine (LAMICTAL), duloxetine hydrochloride (CYMBALTA), ziprasidone hydrochloride (GEODON). On 06Jan2021 at 12:30, The patient experienced heart rate shot up to 170 with numbness of feet, started to feel chest pain, and feeling like she was going to vomit and faint. The events required emergency room visit and were reported as serious per hospitalization. Heart rate at the hospital was 135-140 where two EKG were done and fluids were given. An x-ray of the heart was done and blood work was completed. The patient didn’t receive treatment for the events. The patient didn’t receive any other vaccines within 4 weeks prior to the COVID vaccine. The patient was not diagnosed with COVID-19 prior to vaccination, and has not been tested for COVID-19 since the vaccination. The outcome of the events was resolved in Jan2021. Information on batch/lot number has been requested.; Sender’s Comments: There is a reasonable possibility that the event “”feeling like she was going to vomit”” was related to BNT162b2 based on known drug safety profile. Based on the temporal relationship, the association between the other reported serious events with BNT 162b2 can not be completely excluded. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to regulatory authorities, Ethics Committees, and Investigators, as appropriate.” | |
| COVID19 VACCINE (COVID19) | resident coded on 09Jan at 8am and expired; This is a spontaneous report from a contactable Other Health Professional. A 70-year-old male patient received first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE, Batch/lot number: EL0140), intramuscularly in left arm on 05Jan2021 15:15 at single dose for COVID-19 immunization. Medical history included DM2(Type two diabetes mellitus), CHF(congestive heart failure), open wound, wound infection, heart failure. Allergies to medications, food, or other products: none. Concomitant medications included unspecified products (List of any other medications the patient received within 2 weeks of vaccination: yes). If the patient received any other vaccines within 4 weeks prior to the COVID vaccine: Unknown. Facility where the most recent COVID-19 vaccine was administered: Nursing Home/Senior Living Facility. The resident coded on 09Jan2021 at 8 AM and expired. The patient died on 09Jan2021. An autopsy was not performed. AE resulted in: patient died. Death cause: unknown at this time. Was treatment received for the adverse event: Unknown. Prior to vaccination, was the patient diagnosed with COVID-19: No. Since the vaccination, has the patient been tested for COVID-19: No. Serious: Yes. Seriousness criteria-Results in death: Yes. Seriousness criteria-Life threatening: No. Seriousness criteria-Caused/prolonged hospitalization: No. Seriousness criteria-Disabling/Incapacitating: No. Seriousness criteria-Congenital anomaly/birth defect: No.; Sender’s Comments: The old patient had diabetes mellitus, congestive heart failure, open wound complicated by infection, all these pre-existing medical conditions contribute to the patient death. More information including complete medical history, concomitant medications and event term details especially death cause and autopsy results are needed for a full assessment of the case. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate; Reported Cause(s) of Death: resident coded on 09Jan at 8am and expired | |
| COVID19 VACCINE (COVID19) | Saturday had. Sweating. Chills. Headache. Nausea.; Saturday had. Sweating. Chills. Headache. Nausea.; Saturday had. Sweating. Chills. Headache. Nausea.; Saturday had. Sweating. Chills. Headache. Nausea.; Sunday had emergency appendectomy for actuate appendicitis.; Friday at 3pm, the patient had numbness and tingling to left hand, lips and throat; Friday at 3pm, the patient had numbness and tingling to left hand, lips and throat; Friday at 3pm, the patient had numbness and tingling to left hand, lips and throat; Friday at 3pm, the patient had numbness and tingling to left hand, lips and throat; Friday at 3pm, the patient had numbness and tingling to left hand, lips and throat; Friday at 3pm, the patient had numbness and tingling to left hand, lips and throat; Post surgery had allergic reaction unknown reason with head to toe rash; Post surgery had allergic reaction unknown reason with head to toe rash; This is a spontaneous report from a contactable nurse (patient). A 34-year-old female patient (pregnant: No) received second dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE) via intramuscular (lot number: EL1283) on left arm on 08Jan2021 at 6:30 AM at single dose for covid-19 immunisation. The relevant medical history included celiac, anemia, known allergies: Sulfa and Gluten. Concomitant medications were not reported. The patient received first dose of BNT162B2 via intramuscular (lot number: Ek5730) on left leg on 18Dec2020 at 11:00 AM at single dose for covid-19 immunisation. The patient previously took Codeine, fish oil and experienced allergies. Friday at 3pm, the patient had numbness and tingling to left hand, lips and throat. On Saturday the patient had sweating, chills, headache, nausea. On Sunday had emergency appendectomy for actuate appendicitis. Post surgery had allergic reaction unknown reason with head to toe rash. It was also reported that the adverse event started on 08Jan2021 at 03: 15 PM (as reported). The patient had 1-day hospitalization. The patient received treatment for the events. The adverse events resulted in Emergency room/department or urgent care. The events were reported as serious due to life threatening and hospitalization. The most recent COVID-19 vaccine was administered at hospital. The patient did not receive any other vaccines within 4 weeks prior to the COVID vaccine. The outcome of the events was recovering.; Sender’s Comments: A causal association between BNT162B2 and the reported events cannot be excluded based on a compatible temporal relation between vaccination and onset of events. Medications administered during appendectomy may confound reactions experienced post-surgery. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to RAs, Ethics Committees, and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | “he was in AFIB for about 3 hours after receiving his first dose of the COVID19 Vaccine; he noticed the rest of the day, right up to going to bed that he felt cold; This is a spontaneous report from a contactable consumer. A 66-year-old male patient received first dose of bnt162b2 (PFIZER-BIONTECH COVID-19 VACCINE, Lot Number: EL1284) at arm, left upper, via an unspecified route of administration on 29Dec2020 06:15 at single dose for covid-19 immunisation. Medical history included atrial fibrillation (Afib) formally diagnosed around Jan2020. There were no concomitant medications. The patient reported he got the COVID-19 Vaccine at 06:15 on 29Dec2020 and had to sit for 15 minutes afterwards before he could leave. He said everything was fine, and he walked out to the hospital lobby, and was speaking with a work associate for 10 minutes before he headed to his car. When he got ready to leave, he got in his car, and started his car. He started driving home, and realized he was in AFIB. He did have an AFIB issue. His AFIB has been pretty well controlled for a year now, as long as, he didn’t do something stupid. He had a moderately aggressive run of AFIB. He said his “”smart”” watch told him his heart rate was 117. His normal heart rate is around 60-70. He was in AFIB for about 3 hours. His typical runs of AFIB are considerably shorter. He only had a total of 2 episodes of AFIB in the last year. His prior AFIB episodes would have been about 4-5 minutes. The last time he had AFIB that lasted any length of time was when he was hospitalized for AFIB around Jan2020. He said at that time, he was hospitalized overnight for observation. He received medication during the hospitalization and his AFIB broke. He didn’t remember what medications were given to him during the hospitalization. He said he had flutters before that hospitalization, but the flutters were always gone after a few minutes, so he never sought treatment because there would be nothing to treat. The patient reported he noticed the rest of the day on 29Dec2020, right up to going to bed that he felt cold. His house wasn’t cold, and he didn’t have a fever. He said the cold feeling went away during the night while he was sleeping. He said the cold feeling easily lasted for a 12 hours. He said the cold feeling could have been something else. The outcome of “”he was in afib for about 3 hours after receiving his first dose of the covid19 vaccine”” was recovered on 29Dec2020; of “”he noticed the rest of the day, right up to going to bed that he felt cold”” was recovered on 30Dec2020.” | |
| COVID19 VACCINE (COVID19) | she was diagnosed with bilateral deep vein thrombosis (DVT) and pulmonary embolism (PE); she was diagnosed with bilateral deep vein thrombosis (DVT) and pulmonary embolism (PE); This is a spontaneous report from a contactable nurse (patient). A 22-year-old female patient received 2nd dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE, lot# EK9231), via an unspecified route of administration in left arm on 06Jan2021 13:45 at single dose for COVID-19 immunisation. Medical history included allergy to all fish, and clots. The patient was not pregnant. There were no concomitant medications. The patient previously received 1st dose of BNT162B2 (lot numer: EH9899) in left arm on 16Dec2020 13:45 for COVID-19 immunisation and experienced left sided lower back pain on 20Dec2020. No other vaccine received in four weeks. It was reported that the patient had the first covid vaccine on 16Dec2020 and on 20Dec2020 started with left sided lower back pain and then received the second on 06Jan2021 and then on 09Jan2021 11:00 her legs became blue and swollen and she was diagnosed with bilateral deep vein thrombosis (DVT) and pulmonary embolism (PE). The patient otherwise healthy and had never had covid. Other than the clots, she had no other health issues. The patient underwent lab tests and procedures which included nasal swab: negative on 09Jan2021. Events resulted in doctor or other healthcare professional office/clinic visit, emergency room/department or urgent care, hospitalization, and life threatening illness (immediate risk of death from the event), hospitalized for 2 days (in Jan2021). Adverse event treatment: heparin drip and xarelto at home. Recovered with lasting effects on an unspecified date of Jan2020. This case was reported as serious, serious criteria was life threatening, caused/prolonged hospitalization.; Sender’s Comments: The underlying risk factors/predisposing condition of thrombotic diathesis have been assessed to have played a contributory role toward the events. | |
| COVID19 VACCINE (COVID19) | Resident expired on 12/30/20, dx cardiac arrest. |
| COVID19 VACCINE (COVID19) | Resident expired on 1/2/21. | |
|---|---|---|
| COVID19 VACCINE (COVID19) | Per pt, sx onset began at 1520 with pruritus/hives of the scalp. She was in the post vaccine observation area at this time. At 1530, EE returned to vaccination room to alert staff of her reaction. Upon hearing her new onset cough, an assessment was performed immediately. Reported tingling and swelling of her lips, cough, minor difficulty breathing with mask on, facial flushing and feeling hot, and severe pruritus, especially on the scalp. 50 mg IM Benadryl administered and was taken to ED via wheelchair which is 7 minutes away. Epi Pen administered in ED and admitted overnight for observation d/t irregular HR and ST depression on monitor. | |
| COVID19 VACCINE (COVID19) | Tufts of my hair came out by the handful – first time in my life I have experienced ANY hair loss! It is still ongoing and I am worried it will result in permanent baldness. | |
| COVID19 VACCINE (COVID19) | Cardiac arrest within 1 hour Patient had the second vaccine approximately 2 pm on Tuesday Jan 12th He works at the extended care community and was in good health that morning with no complaints. He waited 10-15 minutes at the vaccine admin site and then told them he felt fine and was ready to get back to work. He then was found unresponsive at 3 pm within an hour of the 2nd vaccine. EMS called immediately worked on him 30 minutes in field then 30 minutes at ER was able to put him on life support yet deemed Brain dead 1-14-21 and pronounced dead an hour or so later | |
| COVID19 VACCINE (COVID19) | Patient died on 1/21-2021 | |
| COVID19 VACCINE (COVID19) | 1/7/2021 @ 5:00 a.m. patient woke up and couldn’t move her right side (neck, arm and leg). Sister helped dress her and drove her to the hospital. During the ride the hospital the patient had 2 seizures and a 3rd seizure in the ER at the hospital. Patient states she has never had multiple, back to back seizures before. States she hasn’t had a seizure for 2 years. EEG was done. Was released from the hospital on 1/9/2021 and to have outpatient MRI of head, shoulder, c-spine. Does not have full range of motion of right neck, and arm, hand and fingers and is painful. 1/14/2021 – full release back to work on 1/14/2021. | |
| COVID19 VACCINE (COVID19) | Resident had been monitored and had shown no signs or symptoms of any kind until 2 pm on 1/14/2021. Resident was found in the floor of her room. She had fallen and was having a seizure, temperature was 99.7F and Oxygen saturation was 82%. | |
| COVID19 VACCINE (COVID19) | Resident found unresponsive and without pulse at 05:45am. | |
| COVID19 VACCINE (COVID19) | on 1/14/21 patients HR 155 at 0800 per patient’s home pulse ox device. arrived at ER, HR 148 at 1130. Continued to stay up even after 2 doses of 10mg iv push Cardizem and one dose of 30mg Cardizem. Cardizem drip started, heparin drip started, patient admitted to hospital | |
| COVID19 VACCINE (COVID19) | “12/23/2020: 2 hr after injection, patient noted swollen lymph nodes, nausea, room spinning (motion sickness-like) sx. Stayed home from work that day and slept. 12/24/2020: “”typical injection site pain”” 12/30/2020: injection site hot, itchy, welts 12/31/2020: area of welts doubled in size to entire upper left arm; throat starting to close up” | |
| COVID19 VACCINE (COVID19) | Patient presented herself to LPN slurring words and ‘not herself’. Upon evaluation, patient denied drinking alcohol, knew she was not able to speak correctly and visibly frustrated . With great difficulty she was able to communicate that she had a headache and was slightly dizzy. Failed FAST and does have a history of CVAs. EMS called and patient was taken to ER where they admitted her for observation post Stroke. Per the hosp nurse, patient received tPA treatment and will be moved to step-down unit when a bed is available. | |
| COVID19 VACCINE (COVID19) | On 1/11/21 noted with headache, nausea/vomiting, severe melaise. On 1/12/21 resident expired. | |
| COVID19 VACCINE (COVID19) | Vaccination given 1314 and sent to waiting room for monitoring. Began to have itching at 1325. PO benadryl administered. Then with throat swelling. Epinephrine administered by EMS/Fire at 1:32pm: 0.5mg IM right arm. 1342 improving 1350 itching/throat swelling returning while EMS/Fire on phone with medical director. 1352 second dose of epinephrine administered by De Pere EMS/Fire: 0.5mg IM left arm Medical Director on site for evaluation. Client given option to transport to hospital or stay for monitoring with EMS/Dr. Condition improving, chose to stay for monitoring. Client improved and up walking halls 1513 Client cleared to be released home via private transport | |
| COVID19 VACCINE (COVID19) | EXTREME LETHERGY, NAUSEA, REFUSING TO EAT OR DRINK, ELEVATED HEARTRATE, FATIGUE, ELEVATED TEMP | |
| COVID19 VACCINE (COVID19) | Day after vaccine : mild shortness of breath, sensation of swelling in my throat/neck area. Took Benadryl 50mg before bedtime. 2 days after vaccine: woke up with voice changes, coughing/choking with speaking. Used epipen once, felt full relief for about 1-2 hours. Trouble speaking again. Then went to ER, had epipen again twice, over two hours, Benadryl 50IV and Pepcid and steroids. Sitting in the ER now debating admission. Likely being admitted., home epipen are too expensive to treat q2h by myself. | |
| COVID19 VACCINE (COVID19) | Generalized myalagias, weakness, vertigo, nausea with emesis, one episode of urinary incontinence. Admitted for observation. Patient improved without intervention with some residual dizziness on day two of symptoms. | |
| COVID19 VACCINE (COVID19) | Dec 24 felt light headed and loss of appetite, Dec 25, fever 103 and chills, Dec 26 Team member Covid hub had me do a Covid test, negative result, continue high fever, no appetite, chills and body ache, Health Hub contacted me daily with app to review symptoms, Dec 27-29 continued symptoms, unable to eat and little bit of water intake, Dec 29 appointment made with Health Urgent Care, received text from Urgent Care cancelling appointment and instructed to take sips of water and Tylenol that I was having an immune reaction to vaccine. Dec 29 virtual appointment with PCP continued symptoms, Dec 31 repeat of COVID swab along with Flu swab both negative, Jan 2-3 hospital admission for abnormal labs, jaundice, dehydration, Sodium/Potassium and Magnesium boluses given, and work up for infectious process was negative. Discharged to home Jan 3 with low grade fever, dry mouth, dry eyes, nausea, body ache and continued loss of appetite. Jan 4-7 continued worsening jaundice and appetite with low grade fever, GI Clinic consulted at Health Clinic, PCP in daily contact, repeat liver function labs on Jan 8 showed worsening labs. Jan 9-12 afebrile, started Ursodiol, low appetite better energy, continue dry mouth, GI appointment Jan 12 with plan to repeat liver functions on Jan 15, jaundice improved and able to eat liquids. No Tylenol or antipyretic taken since Jan 3 due to liver function tests. | |
| COVID19 VACCINE (COVID19) | Developed chest pressure 8.5 hours after vaccine, unrelieved after 3 hours, went to ED, elevated troponin, EKG changes. Admitted to hospital low grade fever next day | |
| COVID19 VACCINE (COVID19) | I developed severe abdominal pain 3 days after injection that turned out to be pancreatitis | |
| COVID19 VACCINE (COVID19) | 71yo female resident who died after receiving Pfizer BioNTech vaccine. On 1/14/2021, VS taken at 10am, B/P 99/60, O2 sats, 95% (trach w/O2). At 11:30am, Patient showed no s/sx of distress, A&Ox3. At 11:50am, a nurse went to perform a COVID test and assessment (the facility is experiencing an outbreak), and found the patient unresponsive on the bathroom floor. CPR was immediately started; no shock advised per AED; 12:15pm EMS arrived and took over. At 12:38pm, EMT called time of death. | |
| COVID19 VACCINE (COVID19) | Has underlying dementia and often with difficulty eating. 1 week after immunization she developed a stroke with left sided weakness and difficulty swallowing. Comfort measures instituted. Not sure if this is related to the vaccine, but thought I should report | |
| COVID19 VACCINE (COVID19) | “83yo female resident who died after receiving Pfizer BioNTech vaccine. On 1/14/2021, the patient reportedly got up in the middle of the night with c/o feeling “”blah””, restlessness, and nausea. VS normal, no other s/sx. At 4:15am, the patient was asked to go back to bed, assisted by a nurse and GNA. At 6am, GNA was going to do morning VS and found the patient unresponsive, no pulse, no respirations. GNA notified the nurse. At 6:03am, CPR started and EMS called. At 6:15am, EMS arrived and took over. At or around 6:30am, EMT called time of death” | |
| COVID19 VACCINE (COVID19) | I had fatigue, headache, pain, weakness and I was so miserable decided to go to ER on (12/24/20) and then was transferred to hospital admitted (12/25/20) one day – discharged on 12/26/20 at night | |
| COVID19 VACCINE (COVID19) | Staff reported that he wasn’t being himself. He was leaning more towards the right. Had symptoms similar to Bell’s Palsy, some right sided facial droop, right eyelid drooping. On CT right maxillary sinusitis, ventriculomegaly. | |
| COVID19 VACCINE (COVID19) | Shortness of breath Chest pain Ongoing since | |
| COVID19 VACCINE (COVID19) | Fever to 100.4 on day 1 after vaccine, to 101.9 on day 3 after vaccine. Acute kidney injury (creatinine rose from 1.73 to 2.43) requiring hospitalization. | |
| COVID19 VACCINE (COVID19) | After first vaccine i experienced fatigue, body aches, headache and nausea for 2 days and injection site pain for two weeks. After second Vaccine given at 9:55am. tingling of feet for about 20 mins, 30 mins after the vaccine. Two hours later fatigue, body aches, headache and nausea began. At 0030 1/12/2021 I woke up with severe chills and left chest pain, temp was 101.6 and heart rate was 160. I began to see black and chest pain was severe feeling like i was having a heart atack and was going to pass out so i called 911. I then began to get short of breath and got numbess on my legs, left arm and left side of neck. Chest pressure/pain radiated to left arm and neck. I was taken by the ambulance to hospital. Arrived around 0130. My heart rate sustained at 140s in the ER so I was admitted at 0530 am. My D Dimer was a bit elevated as well as my lactic so I was given a bolus of fluids plus maintenance. I had a CXR done, CTA chest(negative for PE), UA, Labs, entire cardiac workup including an ECHO during my admission. I also began to have loose stools and wrist joint pain. MY heart rate sustained at 125-147 for about 30 hours. Fevers on and off. After everthing was negative we determined this was secondary to the covid 19 vaccine. I was discharged 1/13/2021 at around 1:30 pm. My heart rate is still not at baseline which is 87-90. I’m 100-130 and still get very fatigued with a simple slow walking. Still having tachycardia, fevers, body aches, joint wrist pain, chest discomfort and headaches. My potassium was 3.0 at discharge so will be needing labs in a week. Also bruised a little different than I usually do with lab draws so keeping an eye on them and will be checking my platelets again in a week. Ive been taking tylenol for my fever and pain and ativan for any anxiety when my heart rate goes up. (treatment during hospital stay was normal saline bolus, nomal saline maintenance fluids at 150 cc/hr, tylenol, ativan, ice packs, rest) | |
| COVID19 VACCINE (COVID19) | Fever, joint pain, weakness. Pain at the injection site. | |
| COVID19 VACCINE (COVID19) | No reactions immediately after vaccine was given. Resident has dementia, has had multiple hospitalizations related to a renal stone recently. Had a tooth that was bothering her, went to see her dentist and it was extracted on 1/6/21. On 1/10 they noted feet and ankles are dark purple with white splotches appears to be mottling. Minimally responsive to voice and touch. Not eating. Compassionate visit with family. Family did not want hospice, did not feel it was needed, said, what more could they do for her than you’re already doing? On 1/11 at 1950 was determined to be deceased. | |
| COVID19 VACCINE (COVID19) | Anaphylaxis- throat tightness , nausea , rash , pruritis , chest tightness, wheezing . 9-11 called epinephrine x 2 , decade on , IV Benadryl , duo-nebs, famotidine, admission to icu high dose prednisone , nebulizers , zofran , duo-neb nebulizers | |
| COVID19 VACCINE (COVID19) | Had no immediate issues with the vaccine. He had returned from the hospital on 12/21 and had some concerns about his weight which were shared with his physician on 1/4/21. On 1/5/21 had a visit with his cardiologist for a pacemaker check. On 1/8/21 staff were called to his room, he was on the floor, bluish skin color. No vital signs found, no heart rhythm heard at 2200. | |
| COVID19 VACCINE (COVID19) | “heart failure; Death; feeling sick; changes with speech and mobility; changes with speech and mobility; This is a spontaneous report from a contactable consumer received from the regulatory authority. The regulatory authority report number is GB-MHRA-WEBCOVID-20210111094207, Safety Report Unique Identifier: GB-MHRA-ADR 24577774. A 97-year-old female patient received the bnt162b2 (PFIZER-BIONTECH COVID-19 MRNA VACCINE; Lot Number: EJ1688), via an unspecified route of administration on 08Jan2021 at a single dose for COVID-19 immunization. The patient’s medical history and concomitant medications were not reported. On 10Jan2021, the patient experienced feeling sick (medically significant), changes with speech and mobility (speech disorder) (medically significant). On 11Jan2021, the patient experienced death (death, medically significant). On an unspecified date, the patient experienced heart failure (death, medically significant). The clinical course was reported as follows: “”The resident had got heart failure.”” The patient was feeling sick on 10Jan2021 and was concerned as there were changes with speech and mobility. Emergency was called, and the ambulance arrived. It was stated the sats were low and blood pressure was low. The ambulance crew called for an out of hours general practitioner (GP) to come and see the patient. The out of hours general practitioner (GP) visited on 10Jan2021 and advised “”she maybe poorly due to having the Covid-19 vaccine”” that was administered on the 08Jan2021. The resident passed away at 07:20 on morning 11Jan2021. The patient had not tested positive for COVID-19 since having the vaccine. The patient had not had symptoms associated with COVID-19. The patient was not enrolled in a clinical trial. The patient underwent lab tests and procedures which included COVID-19 virus test: no-negative COVID-19 test on an unspecified date, oxygen saturation (sats): low on 10Jan2021, blood pressure: low on 10Jan2021. The clinical outcome of the event, death and heart failure, was fatal. The clinical outcome of the event, feeling sick and changes with speech and mobility, was unknown. The patient died on 11Jan2021 due to heart failure. It was unknown if an autopsy was performed. No follow-up attempts are possible. No further information is expected.; Reported Cause(s) of Death: heart failure” | |
| COVID19 VACCINE (COVID19) | started with left sided lower back pain; This is a spontaneous report from a contactable Nurse (patient). A 22-year-old female patient received the first dose of BNT162B2 (lot number: EH9899), via an unspecified route of administration at left arm on 16Dec2020 13:45 at single dose for covid-19 immunization. Medical history included allergies for All fish. The patient’s concomitant medications were not reported. The patient had the first covid vaccine on 16Dec2020 and on 20Dec2020 started with left sided lower back pain. The event resulted in Doctor or other healthcare professional office/clinic visit, Emergency room/department or urgent care, Hospitalization (2 days), Life threatening illness (immediate risk of death from the event). The patient received the Heparin drip and xarelto at home for the event. The patient was not pregnant. The patient received the covid test post vaccination on 09Jan2021. Test type was Nasal Swab. The result was negative. The outcome of the event was recovered with sequel on unspecified date.; Sender’s Comments: From the information provided it is unclear what is the nature of the reported event and what are the reasons that have put the subject at immediate risk of death. The event is considered possibly related to the suspect product based on the positive temporal association. | |
| COVID19 VACCINE (COVID19) | throat closing up; struggled the breath; This is a spontaneous report from a contactable consumer (patient). A 44-year-old female patient (non-pregnant) received first dose of bnt162b2 (PFIZER-BIONTECH COVID-19 VACCINE, Batch/lot number: Pfizer EL 3302), via an unspecified route of administration on 09Jan2021 07:30 am at single dose at left arm for covid-19 immunization. Medical history included diabetes, high blood pressure, allergies. The patient’s concomitant medications were not reported. Patient didn’t receive any other vaccines within 4 weeks prior to the COVID vaccine. The patient was not diagnosed with COVID-19 prior to vaccination. Since the vaccination, patient has not been tested for COVID-19. On 10Jan2021 14:30, patient woke up with throat closing up and struggled the breath. Patient immediately drank a dose of diphenhydramine hydrochloride (BENADRYL). Patient did that two more time in the evening of 10Jan2021. Patient called the doctor in the morning. Events were considered serious per life-threatening. The adverse events resulted in doctor or other healthcare professional office/clinic visit, life threatening illness (immediate risk of death from the event). Patient received treatment liquid diphenhydramine hydrochloride, epinephrine (EPI-PEN) for events. Outcome of events was recovered in Jan2021. | |
| COVID19 VACCINE (COVID19) | Moderna COVID-19 Vaccine At 2 PM I went blind in my left eye. Went to emergency room at Hospital Was told I have Blood clot in my eye causing the blindness and Ophthamologist says it will probably be permanent | |
| COVID19 VACCINE (COVID19) | At approximately 10:30pm on 1/14/2021, resident was noted to have a rash on her face, hands, arms, and chest. VS:100.2, 113, 20,108/59, 84% room air. applied nasal cannula at 4-L, telephoned Physician orders 6mg Decadron one time order, a second set of Vitals , reads 99.3, 110, 20, 106/60, 90% on 4-L N/C. On coming shift advised. At approximately 2:00am on 1/15/2021, resident congested and coughing. BP 151/70, pulse 124, temp 98.1 forehead, resp 20 and pulse oc 79% on 3L. At approximately 2:30am PRN cough syrup and breathing tx. Resident’s condition began to worsen with breathing tx. This LPN updated at 0248 doctor on resident’s condition. Doctor gave permission for resident to go to hospital. At 4:19am the Er called to say resident passed away. | |
| COVID19 VACCINE (COVID19) | THAT EVENING BETWEEN 10-11 PM HAD A GRAND MAL SEIZURE AND WAS UNRESPONSIVE. TAKEN TO ER WHERE BLOOD WORK WAS DONE AND CT SCAN. SENT HOME AROUND 6:30 AM ON JAN 1ST. (I DO NOT REMEMBER MUCH JUST WHAT MY FAMILY HAD TOLD ME) . I DO REMEMBER BEING SEVERLY SICK WITH VOMITING IN THE ER. CURRENTLY FOLLOWING UP WITH MY FAMILY MD AND HAVE MRI AND EEG TESTS SCHECULED WITHIN THE NEXT COUPLE OF WEEKS. | |
| COVID19 VACCINE (COVID19) | 51 year old M with h/o O2 dependent COPD, Severe pulmonary fibrosis became increasingly hypoxic around 1800hours 1/7/2021. He was transported to hospital for acute on chronic hypoxia respiratory failure. On 1/12/2021 he decompensated further, and after discussing with family and palliative care, He was changed to comfort care. He expired on 1/12/2021@2325 at medical center. | |
| COVID19 VACCINE (COVID19) | Maybe 1 minute after receiving the vaccine I began to have a syncopal episode, the nurse practitioner thought that since I was heavy I began to have a vasovagal reaction. From there they asked me to sit with the others waiting 15 minutes to make sure they were ok to leave. As I sat the symptoms would come and go, as if in a pattern, and then return. The longer I sat there the more the symptoms began to grow, after sitting for an hour they decided to send me to the ER. At this time I was experiencing nausea, vomiting, sweating, I was itchy, I could not stop coughing, it was difficult to breath, and I had the worse headache. When I got to the ER they gave me drugs to reverse the allergic reaction and told me they would watch me for a while. In the next 4 hours all of the symptoms started again and I had to get another round of the reaction drugs, I ended up going through this process 4 times before I was safe enough to go home the next day. The provider that saw me and admitted me did not do any blood work or labs, they simply provided me with the reaction drugs when needed and my home medications. | |
| COVID19 VACCINE (COVID19) | Increased lethargy on 1/14/21 at 9pm, Vital signs-106/66, Heart rate-112, temp 98.2; Sent to ER for eval admitted with fever. | |
| COVID19 VACCINE (COVID19) | On 1/8 she took her 2nd dose of Pfizer vaccine around 3 PM. She went home in the evening and started sweating. She passed out. Her daughter was at home and she took her to the nearest hospital. Her BP was 60/34 and she had a temp of 100.3F on 1/8 during hospital admission. Her K was low. She received lot of fluids. She called our office on 1/10 and during the f/u she was still in the hospital. On 1/10 she has a temp of 98.4F and BP of 109/61. But she still has dizziness, nausea, anorexia and mild cough (Former smoker). COVID-19 test was done on 1/9, its negative. Dx was dehydration. Discharged from the hospital on 1/11, feels better but is tired. Will follow up with PCP on 1/16/21. | |
| COVID19 VACCINE (COVID19) | anaphylaxis by lethargy, nausea, vomiting, palpitations, funny feeling in chest, swollen lips | |
| COVID19 VACCINE (COVID19) | “Patient received Pfizer COVID-19 vaccine without any immediate complication on 1/14/21approx 1455, was then escorted to observation area for a 30 minute observation time. Pt had previously had a known reaction to contrast media. Approximately 5 -8 minutes into observation, pt had one audible cough. Nurse asked patient if this was a new onset cough. The patient stated she would try to “”manage”” cough. Pt escorted to bay for monitoring. Pt developed shortness of breath and wheezing rapidly. Rapid response team called and local 9-11 also called. Pt received albuterol nebulizer treatment, placed on O2 at 8L. O2 sat 99%, HR 115-120. Respiratory therapy assisted and Rapid Response Team monitored pt while waiting for EMS. Physician order to give Epinephrine 0.3 mg IM in right deltoid, given as directed at approx 1515. Second epinephrine 0.3 mg IM given approx 1530-1535. HR 144, O2 sat 99%. Patient transported to local ER, pt intubated approx 1927.” | |
| COVID19 VACCINE (COVID19) | Received the 2nd vaccine at 10am on 1/11/21 intramuscular in the right arm. At 3pm on the same day, I had a painful swolle lymph node on left side of neck. That same evening I developed pain, swelling, in my right armpit radiating to the right upper breast and down my right arm with a swollen lymph node under the right arm pit. The pain was about a number 7 on a scale of 1 to 10. The pain and swelling still persist today on 1/15/2021 Still painful, especially to touch. Still radiating down the arm. Lymph node still swollen The pain is about a 2 on a scale of 1 to 10 | |
| COVID19 VACCINE (COVID19) | Rash, swollen tongue and 2 seizures. Admitted to hospital with diagnosis of seizure and allergic reaction., | |
| COVID19 VACCINE (COVID19) | 7:00PM fatigued, burning up fever 100., ibuprofen/tylenol dose; Sunday afternoon nausea, loss control of body, anxious, feeling of fainting, unable to move-paralyzed, pressed button for medical help, ambulance arrived, pt transported to ER — 102. temp ambulance, RN at hosp temp 98., pt was shaky, 8:30PM erratic heartbeat per admitting doctor – pt admitted. Pt PCP/Cardiologist contacted, kept on heart monitor, pt discharged Monday afternoon. 1/14/21 chest pain, nausea, 102. fever. symptoms | |
| COVID19 VACCINE (COVID19) | #Right parietal/temporal subarachnoid hemorrhage and right intra-axial hemorrhage CT brain (1/12/21): Right parietal intra-axial hemorrhage toward the convexity measuring 2.3 x 1.1 x 1.7 cm with decompression into the subarachnoid space, mild right predominantly temporal and parietal subarachnoid hemorrhage is seen with minimal associated hemorrhage along the tentorium. Mild diffuse right cerebral sulcal effacement with minimal leftward midline shift measuring 2.5 mm. #Dural sinus thrombosis CTA head (1/11/21): Increased density within the superior sagittal sinus, inferior sagittal sinus, and transverse sinuses on noncontrasted images with no flow seen on postcontrast sequences consistent with venous sinus thrombosis #Left sided weakness 2/2 above #Recent jaw alignment procedure | |
| COVID19 VACCINE (COVID19) | Patient developed headache and nausea on 1-11-2021. She was hospitalized on 1-14-2021 at Hospital. Found to have dural sinus thrombosis of the superior sagittal and right transverse/sigmoid sinus on MRV brain. Currently admitted to ICU at Hospital, getting injectable blood thinners. Neurology and hematology have been consulted. | |
| COVID19 VACCINE (COVID19) | Hospital Course: á Patient is a 43 y.o. female patient who originally presented to the hospital on 1/3/2021 due to Left lower extremity pain and swelling. Patient found to have extensive DVT of left lower extremity and started on heparin drip. Vascular was consulted and recommended thrombolysis. Patient was also seen by IR who took patient for thrombectomy and left iliac stent placement on 01/05/2021. Patient tolerated procedure well. Patient was transitioned from heparin drip to Eliquis upon discharge. Patient given vascular follow-up as well as Hematology follow-up. | |
| COVID19 VACCINE (COVID19) | Sudden death 18 hours post vaccine . | |
| COVID19 VACCINE (COVID19) | Onset of shortness of breath and cough on 1/3 that progressively got worse. Clinical diagnosis of pneumonia without fever was made, patient started azithromycin on 1/5 and albuterol treatments every 4-6 hrs. Initially he improved, but then worsened. chest xray on 1/6 was negative for pneumonia, PCR covid test was negative, albuterol treatment did not bring much relief. He started respiratory distress on 1/10 and was taken by car to the local ER where another covid test was negative and chest CT revealed multiple bilateral pulmonary emboli. The leg US revealed blood clots in both of his legs. He had an emergency catheter-delivered thrombolysis and was discharged home from the ICU on 1/12 on oral anticoagulants. He is gradually improving, but very weak. He tires easily and gets a drop in oxygen to 90- 93%, as well as an increase in the heart rate to 120 when walking less than half a mile. He runs out of breath with exertion. | |
| COVID19 VACCINE (COVID19) | Patient developed a hoarsenss of voice and tightness of throat and flushed feeling immediately following vaccination. Epi Pen was administered and 50 mg Benadryl given p.o., EMS transport to ED after administration of solumedrol 125 mg – received Pepcid and Zofran and NS IV in the ED. Discharged from ED with prednisone 40 mg daily x 4 day with Epi Pen prescription. | |
| COVID19 VACCINE (COVID19) | Resident received Moderna vaccine on 12/23/2020 around 5 pm. At approximately 3:35 am on 12/25/2020, resident had a CVA and died on 1/1/2021 at 3:00 am. | |
| COVID19 VACCINE (COVID19) | patient died while there were no other complaints at that time; This is a spontaneous report received from a contactable consumer (Pfizer colleague). A 99-year-old female patient received bnt162b2 (COMIRNATY), via an unspecified route of administration on 07Jan2021 at single dose for covid-19 immunization. The patient’s medical history and concomitant medications were not reported. The patient died 3 days after the vaccination while there were no other complaints at that time on 10Jan2021. The patient died on 10Jan2021. It was not reported if an autopsy was performed. The family assessed there was a causal relationship with the vaccine. Information on the lot/batch number has been requested.; Reported Cause(s) of Death: died 3 days after the vaccination while there were no other complaints | |
| COVID19 VACCINE (COVID19) | Died in sleep; This is a spontaneous report from a contactable physician. This is a report received from the regulatory authority. Regulatory authority report number was GB-MHRA-ADR 24556999 with Safety Report Unique Identifier of GB-MHRA-WEBCOVID-20210105122200. An 85-year-old male patient received BNT162B2 (PFIZER-BIONTECH COVID-19 mRNA VACCINE; Lot Number: EJ1688), via an unspecified route of administration on 31Dec2020 as a single dose for COVID-19 vaccination. Medical history included craniotomy in 2019, acute subdural haematoma in 2019, and ongoing bedridden following a craniotomy for an acute subdural haematoma from 2019. The patient was not enrolled in clinical trial. Concomitant medications included atorvastatin (MANUFACTURER UNKNOWN), cetirizine (MANUFACTURER UNKNOWN), ferrous sulfate (MANUFACTURER UNKNOWN), finasteride (MANUFACTURER UNKNOWN), flucloxacillin (MANUFACTURER UNKNOWN), colecalciferol (FULTIUM D3), gabapentin (MANUFACTURER UNKNOWN), hypromellose (MANUFACTURER UNKNOWN), levothyroxine sodium (MANUFACTURER UNKNOWN), betamethasone dipropionate/clotrimazole (LOTRIDERM) , macrogol (MANUFACTURER UNKNOWN), tramadol hydrochloride (MAROL), omeprazole (MANUFACTURER UNKNOWN), oxybutynin (MANUFACTURER UNKNOWN), paracetamol (MANUFACTURER UNKNOWN), senna spp. (MANUFACTURER UNKNOWN), and influenza vaccine inact sag 4v (FLUCELVAX TETRA). On 05Jan2021, the patient died in his sleep. The clinical course was as follows: The patient had not had symptoms associated with COVID-19. The patient received the vaccination on 31Dec2020. The patient had tested negative for COVID-19 since having the vaccine on an unknown date. There were no other reactions noted but the patient died in his sleep overnight on 05Jan2021. It was not reported if an autopsy was performed.; Reported Cause(s) of Death: Died in sleep | |
| COVID19 VACCINE (COVID19) | tested Covid positive/suspected COVID-19; tested Covid positive/suspected COVID-19; Shortness of breath; Fall; This is a spontaneous report from a contactable physician from the Regulatory Agency. The regulatory authority report number is GB-MHRA-WEBCOVID-20210106123053. An 81-year-old male patient received his first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE) via an unspecified route of administration on 19Dec2020 at single dose for COVID-19 immunisation. Medical history and concomitant medications were not reported. The patient experienced SARS-CoV-2 infection, shortness of breath on 03Jan2021. Reaction to vaccine is none. Patient was admitted with fall and on the floor for 5 hours on 03Jan2021. He was tested COVID positive on admission on 03Jan2021. So he tested positive about two weeks after first dose of Pfizer COVID-19 vaccine. Patient was suspected COVID-19 from 03Jan2021. The patient underwent lab test included COVID-19 virus test: Yes – Positive COVID-19 test (03Jan2021). Outcome of the events was fatal. The patient died on 03Jan2021. It was unknown if an autopsy was performed. Cause of death reported as SARS-CoV-2 infection/suspected COVID-19, shortness of breath and fall. No follow-up attempts possible. Information about lot/batch number cannot be obtained.; Reported Cause(s) of Death: SARS-CoV-2 infection/suspected COVID-19; SARS-CoV-2 infection/suspected COVID-19; shortness of breath; Fall | |
| COVID19 VACCINE (COVID19) | spontaneous rupture of membranes at 36-0 weeks; Pregnant at the time of vaccination?: Yes; This is a spontaneous report from a contactable physician. This physician reported information for both mother and fetus/baby. This is the maternal report. A 29-year-old female patient received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE), intramuscular (left arm) on 18Dec2020 and 08Jan2021 at a single dose for COVID-19 immunization. Medical history included cold sores, no active cold sore at time of labor. On an unspecified date, it was reported that a healthy 29-year-old G1P0 with good prenatal care and no pregnancy complications who had spontaneous rupture of membranes at 36-0 weeks, one day after her second Pfizer CoVid vaccine (09Jan2021). She felt well after her vaccine and had no symptoms today. The mother reported she became pregnant while taking BNT162B2. The patient takes prenatal vitamins. The mother was 33 Weeks pregnant at the onset of the event. The mother was due to deliver on 06Feb2021. Therapeutic measures were taken as a result of premature rupture of membranes. The outcome of the event was recovering. Information about Batch/Lot number has been requested.; Sender’s Comments: Based on available information, a possible contributory role of the subject product, BNT162B2 vaccine, cannot be excluded for the reported event of spontaneous rupture of membranes due to temporal relationship. However, the reported event may possibly represent intercurrent medical condition in this patient. There is limited information provided in this report. Additional information is needed to better assess the case, including complete medical history, diagnostics, counteractive treatment measures and concomitant medications. This case will be reassessed once additional information is available. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | short of breath; aching so bad again; was still short of breath, getting worse, aching so bad again; choking; got as cold as ice; throat was real swollen; started itching terribly; This is a spontaneous report from a contactable consumer (patient). A 73-year-old male patient received first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE, lot number EK4176), via an unspecified route of administration on 06Jan2021 single for COVID-19 immunization. Medical history included hypertension and had 4 heart attacks. The patient’s concomitant medications were not reported. The patient just had the Covid 19 Vaccine first shot. After the shot he went to the dining room to get some lunch. He was reading since ‘calculative’. He started itching terribly. Onset date for started itching terribly was reported as 06Jan2021. He got as cold as ice. He started getting real short of breath, he was choking but he managed to get them out of his throat. His throat was real swollen and choked. He went back to the clinic and the Nurse took him straight to the emergency room. He was there in his wheelchair freeze in and crawling himself, struggling to breathe for almost 25 minutes before the Nurse finally came in and helped him get him on the stretcher. She took his time, she got the IV started and gave him a steroid a shot and Benadryl. He did not know the dosage and some capsules. He did not know why the capsules and he was still struggling for a while but he found day tough for about an hour. When he came too he was still short of breath, getting worse, aching so bad again. He was struggling to breathe, he was still having some breathing problem, some choking and aching. The events was still short of breath, getting worse, aching so bad again were serious as hospitalization. Laboratory work: Work was normal. The COVID Test was negative. The outcome of events short of breath and started itching terribly was not recovered while for other events were unknown. | |
| COVID19 VACCINE (COVID19) | died two days after receiving the vaccine; Fever; This is a spontaneous report from a contactable consumer (patient’s stepchild). A 66-year-old male patient received the second dose of BNT162B2 (PFIZER-BIONTECH COVID-19 MRNA VACCINE), via an unspecified route of administration, on 07Jan2021 (at the age of 66-years-old) as a single dose for COVID-19 immunization. The patient’s medical history was not reported. Concomitant medications included an unspecified statin. The patient experienced fever on 08Jan2021. The patient died two days after receiving the vaccine on 09Jan2021, which was reported as fatal. The clinical course was reported as follows: The patient had a fever the day after getting the vaccine and then he just died in the middle of night. It was reported that it was not clear what exactly happened, but they are looking into this. The clinical outcome of fever was unknown and of died two days after receiving the vaccine was fatal. The patient died on 09Jan2021. The cause of death was not reported. An autopsy was not performed (was reported to be taking place soon). The batch/lot number for the vaccine, BNT162B2, was not provided and has been requested during follow up.; Reported Cause(s) of Death: died two days after receiving the vaccine | |
| COVID19 VACCINE (COVID19) | Pt. with dizziness, then Afib with RVR, then massive cerebral hemorrhage Pt. non oriented & unable to give history – History provided by S.O and daughter | |
| COVID19 VACCINE (COVID19) | Accelerated decline in condition with decreased input, decreased responsiveness, somnolence, and death | |
| COVID19 VACCINE (COVID19) | Received vaccine in left deltoid within minute felt throat tighten self administered personal epi pen. | |
| COVID19 VACCINE (COVID19) | I had no side effects after my vaccine on 12/24/20 until 1/8/21. On Friday, 1/8/21 at 830pm I began with severe abdominal pain, low grade fever, nausea and loss of appetite. My abdominal pain persisted and worsened over the next 24-36hours. I presented to the ER on Sunday, January 10, 2021 at 8am with severe right lower quadrant pain, pelvic pain, nausea and low grade fever. I was promptly diagnosed with appendicitis and taken to the OR at approximately 2pm on the same day. In the OR my appendix was gangrenous, there was pus in the pelvic area nd fluid in my peritoneum. My appendix was not ruptured. My appendix was removed as well as part of the omentum. I remained in the hospital on IV Metronidazole and Ciprofloxacin for 2 days and was discharged on 1/13/21 at 9pm. I am continuing to recvoer at home on the same 2 antibiotics in oral form. I have a JP drain that is still in place. Of note I had two negative COVID 19 tests on 1/9/21 and 1/10/21. Both were PCR tests. | |
| COVID19 VACCINE (COVID19) | Tingling and throat swelling to Moderna COVID-19 Vaccine EUA | |
| COVID19 VACCINE (COVID19) | Patient had no immediate effects from the vaccine, but died approximately 8 hours after receiving first dose of vaccine. | |
| COVID19 VACCINE (COVID19) | Resident was found without a pulse and not breathing 20 minutes after vaccine administration. Upon MD review, no signs of anaphylaxis were noted. | |
| COVID19 VACCINE (COVID19) | About 10 minutes after getting my vaccine I noticed the roof of my mouth itching as well as my tongue and back of my throat. I waited to see if it would go away and then a couple minutes later noticed my lips started itching and swelling and from there it just got worse. I told the nurse practitioner that I think I was having a reaction, she had me take a seat told her my entire mouth throat & lips felt swollen and itching and she looked and said it was full blown anaphylaxis reaction. Administered EpiPen, benadryl and called ambulance where they took me to medial emergency department. | |
| COVID19 VACCINE (COVID19) | Patient had just recovered from COVID and ended quarantine on 1/1/21. On 1/4/21, she received the first COVID vaccination. While driving home on 1/6/21, she experienced an MI diagnosed in the emergency room 30 minutes later. She was admitted to the hospital and underwent a heart catheterization and received a STENT. She had previous cardiogenic shock in 2003. | |
| COVID19 VACCINE (COVID19) | Pfizer-BioNTech COVID- 19 Vaccine EUA Received communication that patient experienced a stroke and received alteplase at a non-facility (Medical Center) 5 days after receiving COVID-19 vaccination. | |
| COVID19 VACCINE (COVID19) | increase weakness and fatigue, weakness in extremities, incontinent, jerky arm movements, within first 24 hours, continue to decline sent to hospital returned weaker, within 24 hrs hours BP dropped, low pulse oximeter reading, diaphoretic, lung sounds diminished, loss consciousness and passed away. 01-12-2021 | |
| COVID19 VACCINE (COVID19) | 01/11/2021- Found lying on bed in apartment, incontinent, lethargic, unable to respond to questions, unable to do hand grasps. sent to hospital. 01/14/2021- remains in hospital- confusion and disorientation continues, poor verbal skills, limited ability to feed self, out of bed to sit in chair. uncertain of return to facility status. | |
| COVID19 VACCINE (COVID19) | Abdominal pain, Headaches, chest pain, loss of appetite, confusion, elevated liver enzymes 1/8-1/15/21 | |
| COVID19 VACCINE (COVID19) | 01-09-2021 Very confused, nauseous, sweating profusely. sent to hospital- admitted with encephalitis, severe cognition decline. 01-13-2021 still in hospital, cognition Improved. | |
| COVID19 VACCINE (COVID19) | Death Chest pain; irreg heart rhythm; evening of vaccine; death on toilet on 1/13/21 | |
| COVID19 VACCINE (COVID19) | Initially headache and body aches. Within 24 hours developed chest pain, dyspnea on exertion and shortness of breath. Patient still currently hospitalized. | |
| COVID19 VACCINE (COVID19) | Patient reportedly expired the day following receipt of the vaccine. | |
| COVID19 VACCINE (COVID19) | Observed in her room having seizure activity and unresponsive to stimuli. BP of 200/120, oxygen level dropped to 86%, HR was 116. She was transferred from Hospital A and later transferred to Hospital B and placed on a ventilator. This remains her current status | |
| COVID19 VACCINE (COVID19) | We (myself and 2 other pharmacists) were conducting a COVID-19 vaccine clinic. The patient is on staff at the clinic and came in for her 1st dose of the Pfizer/BioNTech COVID vaccine. 10 minutes post-vaccination, patient started experiencing SOB, tingling fingers and face, and swelling of her lips and tongue. She moved herself outside to cooler air and then sent someone back inside to ask us for help. I ran outside with an EpiPen and immediately noted her pulse of 158 on her watch and she appeared to be experiencing an anaphylactic reaction. Patient stated she did not want to use the EpiPen but wanted to try chewing Benadryl instead first. I asked the staff for a blood pressure monitor and pulse oximeter. The 1st readings, approximately 12 minutes after vaccination, were HR 158, BP 155/105, and pulse ox 97%. Patient stated the Benadryl was working and her swelling was decreasing. The patient was not having trouble breathing at the time. I continued monitoring vitals and talking with the patient and approximately 20 minutes post-vaccination, she was improving (BP down to 134/80 and HR 120) but agreed we should call 911. She decided she wanted to move inside and lie down. I escorted her with support to a bed. Her vitals then increased again to BP 152/95 and HR 133 and her lips and tongue started swelling again. The patient appeared to be more labored in breathing then but still refused the EpiPen. Roughly 5 minutes after lying down, the medics showed up and took over and I went back to the vaccination area. I learned later that the patient refused to go to the hospital and after more observation was eventually allowed to leave with a friend/coworker driving her home. | |
| COVID19 VACCINE (COVID19) | Patient was at work on 1/15/21 after having received the vaccine on 1/12/21. She complained of being diaphoretic and her heart rate was 156. She was taken to Hospital. She is being admitted to hospital and has reported that her heart rate is 122 and she has a temperature of 99.4 and elevated lactic acid levels. | |
| COVID19 VACCINE (COVID19) | 1/5/2021 0718 Pt received 2nd COVID vaccine 1/4/21. Oral temp 100.6, near syncope, muscle aches and head aches. Per EMS NSR, denies SOB, and chest pain. 500 ml bolus received in route. In ED, Pt c/o nausea. Pt anxious, sweating and uncomfortable. 0855 Pt resting comfortably, reports decease in pain/body aches 4/10. 1043 Discharge disposition: home. Accompanied By: self. Mode: walk. –Hospital | |
| COVID19 VACCINE (COVID19) | Expired on 1/12/2021; unknown cause of death | |
| COVID19 VACCINE (COVID19) | We got a call from a home health nurse Brandu Talamo, stating that the patient passed away. | |
| COVID19 VACCINE (COVID19) | 5-6 HOURS AFTER VACCINATION. CONVULSIONS/SEIZURE, HIGH BLOOD PRESSURE, INCREASED HEART RATE, | |
| COVID19 VACCINE (COVID19) | Severe pain at injection site with some swelling, lethargy, and fever to 101.6 degrees F the day after the injection. He was given acetaminophen, which reduced fever to normal. Low grade fever (99-100 degrees F) two days after vaccine. On the third day after vaccine, on 12/31, he became confused and anxious. Temperature was 104.8 degrees F. He was given acetaminophen and oral hydration overnight. He continued to have a fever to 102 on the following day and was taken to the ED. He was COVID-positive and admitted to the hospital for treatment of COVID-19. | |
| COVID19 VACCINE (COVID19) | Initially started as shortness of breath followed by fevers, aches, muscle cramps. Ultimately ended up in the hospital with hypoxemia, pleural effusions. Laboratory values showed evidence of acute renal insufficiency, eosinophilia. Physical exam consistent with pulmonary edema and lower extremity macular rash. Entire presentation concerning for DRESS syndrome | |
| COVID19 VACCINE (COVID19) | Resident had lunch on 01/14/21 and after lunch around 2:00pm, he vomited and stopped breathing. We coded the resident and 911 paramedics came. They pronounced him dead at 2:18pm. | |
| COVID19 VACCINE (COVID19) | Around 00:50am on 01/15/21, C.N.A. reported that the resident looked different and not responding. Initiated Code Blue and started CPR. 911 arrived and pronounced resident dead at 1:01 am. | |
| COVID19 VACCINE (COVID19) | “The patient stated “” I just feel Blah””. vital signs obtained. 156/75 p-84 spo2 94% via NC 2L. T-96.7, c/o feeling restless, c/o nausea with no vomiting. Patient observed at 0600 nonresponsive, CPR initiated, and EMS notified Patient expired” | |
| COVID19 VACCINE (COVID19) | Received Pfizer vaccine, first dose on Wed. 01/13/21 between 12 and 1 P.M. Thurs. 01/14/21 in the afternoon he began to note that he had difficultly walking. Went to bed when he woke up at 5:48 A.M. he reported he had ataxia. Patient reported having to walk in tiny steps to stay upright. He went to the emergency room. Had CT scan of head and found blood clots. MRI performed. Stroke found in right PCA territory, but no loss in strength in left lower extremity. Sensation and vision intact. Strength in all four extremities is 5 out of 5. | |
| COVID19 VACCINE (COVID19) | At 6 days after my second COVID-19 Pfizer vaccine (first dose given 12/17/20), I had acute onset of chest pain and shortness of breath prompting a trip to the Emergency Department. A chest CT Angio to rule out pulmonary embolus was done and negative for pulmonary embolus. My EKG showed some mild ST changes and a troponin I level was elevated at 0.08 (normal 0.04). Subsequent troponin levels 90 minutes apart showed a rising troponin at 0.18 and 0.38. An echocardiogram was performed which showed regional wall motion abnormalities consistent with Takotsubo cardiomyopathy and an ejection fraction of 45%. I was then taken to cardiac catheterization lab for coronary angiograms which were normal. My LV angiogram was consistent with Takotsubo cardiomyopathy and my LVEDP was elevated. I was started on a beta blocker and sent home the following day. | |
| COVID19 VACCINE (COVID19) | This patient has been under hospice care for over 2 years at the nursing home. She has had a steady decline with gradual weight loss. She was totally dependent in her care needs. She received the vaccine on 1/2/2021 as part of the facility vaccination campaign. No adverse events noted initially. On 1/3/2021 at 6:06 pm, she was noted on vital sign checks (done every 4 hours for first 72 hours after vaccination) with BP 64/52 but otherwise asymptomatic. Subsequent BP improved. On 1/4/2021 at 4:45 am, pt found with respiratory rate of 30 with otherwise normal vital signs. Tachypnea persisted, so she received liquid morphine 2.5 mg without improvement. Supplemental oxygen was applied. Tachypnea persisted. She had poor oral intake after that point had persistent tachypnea and worsening hypoxemia despite clear lungs on exam. She remained under hospice care and comfort measures were continued. No blood testing or imaging tests were done. She required increasing amounts of oxygen, became hypotensive, and died peacefully on 1/8/2021 at 7:45 pm. | |
| COVID19 VACCINE (COVID19) | Veteran was found by family slumped over and unresponsive at the breakfast table on 1/13/21, had expired | |
| COVID19 VACCINE (COVID19) | Anaphylaxis | |
| COVID19 VACCINE (COVID19) | Pt had 3 vessel CABG on 1/14/21 after presenting to ED with chest pain on 1/9/21. Pt is critically ill following OR after cardiogenic shock, bleeding. Requiring inotropes and Impella. | |
| COVID19 VACCINE (COVID19) | vertigo nausea / vomiting diarrhea low-grade fever pain at injection site | |
| COVID19 VACCINE (COVID19) | Bells Palsy 1st injection on 12/20/21 symptoms started 1/7/21, 2nd dose on 1/10/21 immediate numbness in mouth and tightness in throat which triggered bells palsy symptoms immediately | |
| COVID19 VACCINE (COVID19) | Patient information was reviewed. Patient was asked if they ever had any form of severe reaction to anything they had had in the past. Patient did not state yes to anything other than having a reaction that was managed at home to shellfish as a child. They stated their throat had swollen as a child during the event. Patient was told that it was an anaphylactic reaction and shouldn’t have been managed at home as a child by nurse and should have been taken to the hospital. Patient was told they would be monitored for 30 minutes after receiving the vaccine. Within 2-3 minutes after receiving the vaccine the patient reported tingling or burning around the site of injections. Within 5 minutes they stated to be feeling tired and that their arm had felt numb. After this the patient began starring into the distance and was unresponsive. Called for help and advice by turning around as patient had syncoped. Got nurses and other team members attention and had an epi-pen ready just in case it was needed. Patient began having notable distress with respiration and administed an epi-pen. Advised for a nurse to call EMS for patient and crash cart/oxygen was also obtaine dby the facility. Oxygen was required. Patient was not responsive after the first dose and was given a second as they remained un-alert with notable distress. The patient became alert a while after receiving the second shot and not long after that the EMS arrived to the event. | |
| COVID19 VACCINE (COVID19) | Resident reported loose stool, not feeling well, resident complaint of lost taste. Hospitalization on 01/14/2020. | |
| COVID19 VACCINE (COVID19) | Vomiting | |
| COVID19 VACCINE (COVID19) | “Per husband, was in usual state of health on the AM of 1/10/20, AOx3 able to perform all I/ADLs. At around 2:30pm that day was complaining of chills and generalized malaise. Then at ~9:30pm when husband returned home from work found patient diaphoretic, confused (stating things like “”not now, I want to go to lake””), and complaining of chills and weakness. Unable to provide any additional hx regarding other sx. Initially presented to ED, where mental status had deteriorated to AOx0, unable to respond to verbal commands. Initial vitals notable for T102.6F (unclear other vitals). Patient is now AOx0 most concerning for encephalopathy.” | |
| COVID19 VACCINE (COVID19) | Patient 101 years old, nursing home resident, received vaccine 1/11, on 1/13 found on floor without obvious trauma, unresponsive. Brought to ED and was bradycardic, hypotensive, hypothermic and refractory to aggressive medical management. No obvious cause of death found on exam or labs, cxr. Unknown if event could be related to vaccine or not. Medical Examiner accepted case although initially unknown that patient had recently received vaccine. ME updated with that information today as soon as discovered. | |
| COVID19 VACCINE (COVID19) | Throbbing head ache, difficulty breathing, lips numbness, chest discomfort, upper back, lower legs, fingers tingling/numbness, high blood pressure 148/83, underarm sweating, feels weak | |
| COVID19 VACCINE (COVID19) | Nausea with emesis, headache, upper abdominal pain. | |
| COVID19 VACCINE (COVID19) | Patient suffered a cardiac arrest and was unable to give details about her symptoms. Per husband, patient did not complain of any symptoms after vaccine administration. She began seizing without warning which was complicated by cardiac arrest of uncertain etiology | |
| COVID19 VACCINE (COVID19) | Right arm weakness 2 hrs after vaccine and then right leg weakness later that evening |
| COVID19 VACCINE (COVID19) | “On 1/15/2021 at 1800, resident noted to be lethargic and shaking, stating “”I don’t care.”” repeatedly. C/O head and neck pain. T100.6. Given Tylenol with no relief of pain. Order received for Aleve and administered.. Assisted to bed as usual in evening. Monitored during night shift and noted to be resting comfortably/sleeping.. Noted agonal breathing at 4:10 AM 1/16/2021 , T 99.4, Absence of vital signs at 4:15AM 1/16/21 and death pronounced at 4:40AM 1/16/21.” | |
|---|---|---|
| COVID19 VACCINE (COVID19) | “””Moderna COVID-19 Vaccine EUA”” It has been reported to me that pt. had gone into hospital for a heart catheterization on 1/12/2021. It was found during this procedure that pt. had suffered a MI. She was release to home the following day and passed away at her residence on 1/15/2021.” | |
| COVID19 VACCINE (COVID19) | anaphalactic shock reaction, epi injection by hospital emergency staff at vaccine site, emergency room admission . We were very lucky vaccine site was Hospital was concerned that this might happen as patient had a previous anaphalactic shock by antibiotic injection few years ago | |
| COVID19 VACCINE (COVID19) | Right thumb joint pain. I can’t hold unto things in my right hand that requires me using pressure from my thumb. Pain is a 9/10 when holding, grabbing, or using the thumb in any way. When not using the thumb there is no pain at all. I work out everyday and I am no longer to lift weights using my right hand because of the excruciating pain when I try to. | |
| COVID19 VACCINE (COVID19) | Patient had COVID-19 infection April 2020, ataxia and myoclonus developed, treated with IVIG and steroids at that time. June 2020 had full recovery after PT. Pt received Pfizer COVID-19 dose 1 on 12/22/20 and developed similar symptoms to above on 1/7/2021. Ataxia and tremor on examination, no myoclonus at this time. | |
| COVID19 VACCINE (COVID19) | Appendectomy Narrative: Developed abdominal pain with nausea on 12/24/2020, went to ER and noted to have appendicitis, resulting in an appendectomy | |
| COVID19 VACCINE (COVID19) | Swollen tongue and sob with decreased swallow | |
| COVID19 VACCINE (COVID19) | Vaccinated at 8:55am, Per Nurse Practitioner note patient started experiencing itching upper chest, medicated with Benadryl P.O., started with throat itching, medicated with Benadryl IM, EPI IM, EMS called, placed on O2, diminished breath sounds bases, slight stridor, EPI IM, Solumedrol IM, EMS transported to local ED. Patient called NP later in day and said she was admitted to the hospital. | |
| COVID19 VACCINE (COVID19) | Pt had witnessed arrest by wife. Pt wife started CPR and called EMS. CPR started at 15:12. Continued by EMS. Pt arrived to medical center asystole with CRP in progress and ventilated via igel device. He was in refractory ventricular fibrillation and continued CPR for a total of 1 hour. At that point, we checked a bedside ultrasound which showed his heart at a standstill. He was unresponsive to verbal and tactile stimulus and had fixed unreactive pupils. He was pronounced at 16:13. | |
| COVID19 VACCINE (COVID19) | Tachypnea, Angina, Tachycardia, Sore Muscles Narrative: Started feeling ill on the 28th. Drove himself to get checked out at the hospital. Hospital sent him home. Called EMS at 12/30/2020 @ 0200 and was hospitalized for 1 day. He is more stable now but still has these symptoms at a moderate level. | |
| COVID19 VACCINE (COVID19) | ADVERSE REACTION REPORTED AS TEMPERATURE OF 100.0, CONFUSION, AND VERTIGO. PATIENT WAS TAKEN TO HOSPITAL, UNAWARE OF CURRENT STATUS, TREATMENTS, OR OUTCOME. | |
| COVID19 VACCINE (COVID19) | After vaccination on 1.8.21 felt fatigue, metallic taste, and intermittent tingling. on 1.15.21 at approximately 8 am developed occipital headache (4/10 pain) inability to move right upper or lower extremities and slurred speech. No evidence of intracranial hemorrhage on CT. Patient administered Alteplase and transferred to higher level of care | |
| COVID19 VACCINE (COVID19) | Day 1-3 after the dose flu like symptoms Day 3-7 swelling in lymph nodes on left side of body (baseball sized) took ibuprofen and Tylenol Day 8 angioedema, anaphylaxis. Received epi subq, IVP 50mg Benadryl, Pepcid 20mg IVP, liter of NS Day 9 raised red rash all over body and face still going on Day 16- present: severe joint pain and fever, unable to obtain any relief | |
| COVID19 VACCINE (COVID19) | Muscle Spas; Weakness in both limbs. Pressured speech | |
| COVID19 VACCINE (COVID19) | Pounding headache, heart racing to over 145 bps, chest burning and tightness and hard to breath. I was taken to the Emergency Room at Hospital immediately. Reaction occurred within 30 minutes of the injection. An EKG was administered. I was prescribed prednisone and Benadryl. I was diagnosed with Anaphylaxis. | |
| COVID19 VACCINE (COVID19) | Patient had slow progression of kidney disease but since vaccine had unexpected acute kidney failure. He had to have dialysis and may need biopsy of kidney to confirm if he needs lifelong dialysis. He is still being hospitalized. | |
| COVID19 VACCINE (COVID19) | Numbness and tingling sensations in both hands and sometimes radiating up my forearms, more severe in right hand and right thumb; these symptoms still didn?t go away since 1/11 | |
| COVID19 VACCINE (COVID19) | Death | |
| COVID19 VACCINE (COVID19) | After 2nd dose in the afternoon at 2:30pm pt collapse in the unit V/S was high blood sugar was high. Pt was held over at work for 16 hours. Shot was given at 6:45am. Paramedics was called. Sent to ER. | |
| COVID19 VACCINE (COVID19) | Remarkable Myalgia of extremities and back, interfering with rolling or sitting. Spasmodic twitching of upper extremities, These resulted in Hospitalization. | |
| COVID19 VACCINE (COVID19) | Resident expired | |
| COVID19 VACCINE (COVID19) | On 1/9/2021 started to have Postural Orthostatic Tachycardia Syndrome and PVCs associated with SOB and chest tightness and not recovered yet. | |
| COVID19 VACCINE (COVID19) | Headache after dose was given at 10:00 a.m Died at after 7:30 pm the same night the dose was given. | |
| COVID19 VACCINE (COVID19) | On January 14, 2021, I noticed generalized petechiae all over my body. I went to seek medical care and was found to have platelet count of 2. I was hospitalized for idiopathic thrombocytopenic purapura. I was given platelets which increased my platelets to 4. Next day, given IVIG dose. Also receiving 4 doses of decadron. Day after IVIG, platelets to 20. I am still in the hospital getting treatment today. | |
| COVID19 VACCINE (COVID19) | Moderna COVID-19 Vaccine EUA. Patient has tested positive for Covid 19 as of 1/10/2021, when he was hospitalized. Patient’s wife had been rushed to the ER previously, and they discovered she was Covid positive when she was admitted. Patient immediately notified public health and was being restricted and followed up with. During his quarantine, he started developing symptoms (08Jan2021). On 10 Jan, he was rushed to the ER and diagnosed with pneumonia due to Covid. He was also experiencing painful stomach cramps, low oxygen saturations and fluctuations in blood pressure. He spent 3 days inpatient in the Covid wing then was discharged to resume resting at home. Our provider immediately reached out to the member and checked in with them. At this time, the patient’s only real complaint is lack of energy or getting winded when doing projects around the house. Patient will isolate at home and monitor his symptoms. Public health and the provider will follow up with the member and her family periodically to ensure recovery. | |
| COVID19 VACCINE (COVID19) | PATIENT GOT HER FIRST COVID PFIZER VACCINE AT 12/31 IN THE AM. HAD GOTTEN FLU LIKE SYMPTOMS AND HAD BEEN SICK FOR A COUPLE OF DAYS. HAD NAUSEA AND VOMITTING DURING THIS TIME AS WELL. ON 1/3 THE CARE GIVER WENT TO CHECK ON HER PT AT HER LTC FACILITY WHERE SHE LIVES AND SHE WASN’T ACTING RIGHT. SHE WAS UNABLE TO DO A STROKE EXAM. PT HAD NO MOVEMNET IN ARMS OR LEGS AND WAS UNABLE TO SPEAK. PT WAS VITALLY STABLE AT THE TIME. EMS RECORDED THAT THEY THOUGHT DIAGNOSIS WOULD BE STROKE, PNEUMONIA OR SEPSIS. AFTER ARRIVAL AT THE HOSPITIAL DETERMED THAT SHE HAD A STORKE, ACUTE KIDNEY INJURY, ABNORMAL LFTS. | |
| COVID19 VACCINE (COVID19) | allergic reaction- skin rash , throat itching. patient visited the ER and was given episode and steroids but returned because symptoms were not improving. She was hospitalized on 1/11 for this reason | |
| COVID19 VACCINE (COVID19) | 12/29/2020 Vaccination 12-13 minutes later started left arm was tight and sore. Wasn’t feeling right; hot, hives, rash at injection site up neck and face; tightness in throat; tachychardic, BP increase. I took benadryl and Pepcid . Sat for another 30-45 min. Palpitations, heaviness. Transported to ER. IV and medications. Stayed till 1:30, went home, and then came that evening. 5:00 that same night, hives, chest pressure, burning sensation, ‘didn’t feel right’, anxious. Benadryl. Tachychardic; admitted to hospital that evening. Stayed in house admission till the 12/31/2020. Went back to admission in house, 1/3/2021 – 1/4/2021. Two hospital admissions. | |
| COVID19 VACCINE (COVID19) | Approximately 28 hours after vaccine, I began to feel tingling in my right eye Approximately 12 hours after that, my face started drooping and was numb so I went to ER. Today is Sunday, and the numbness and drooping was called Bells Palsy at the hospital. | |
| COVID19 VACCINE (COVID19) | Injection given without unusual pain, but appeared to be at higher site than usual for other vaccinations patient has received. No immediate reactions. No redness or swelling at injection site. Approximately 3 hours later with restriction of abduction of left arm, which became worse over 24 hours. No numbnness, pain 4-5/10 diffusely over deltoid and in acromium, posterior suprascapular area.. Able to passively move arm, treated with topical Voltaren gel, Naproxyn 500 x 1 dose. D2 with increased ROM, but still restricted. | |
| COVID19 VACCINE (COVID19) | New onset leukopenia/neutropenia with fever, unclear if related to vaccination, but temporally occurred the day after receiving 1st dose. No labs immediately prior to vaccination, so leukopenia may have preceded vaccine. No other new medications to explain neutropenia. Off chemotherapy since 8/2020. Possible relapsed disease though no other evidence in support of this as remainder of CBC stable. | |
| COVID19 VACCINE (COVID19) | Shortness of breath, Congestive heart failure, Afib | |
| COVID19 VACCINE (COVID19) | RespDepression found to have low potassium Narrative: Patient reports experiencing shortness of breath 24 hours after the vaccine was administered, went to ER and admitted for one night. Informed her supervisor she had low potassium. | |
| COVID19 VACCINE (COVID19) | Patient presented with diffuse petechiae, easy bruising and oral bruises. She has a history of stable ITP with her last required infusion of IVIG 12 years prior during pregnancy and monitors her CBC every 6 months. Her baseline platelet count is ~50-60k. She received treatment with dexamethasone 40 mg IVPB x 3 doses and IVIG 95 grams (1 gram/kg) IVPB x 2 doses. | |
| COVID19 VACCINE (COVID19) | 3 days after receiving the first dose of the vaccine on 12/21/2020, experienced flu-like symptoms that lasted 24 hours. About 17 hours after receiving the second does on 1/11/2021, flu-like symptoms reoccurred. The following day (1/13/2021) severe chills, and difficulty breathing occurred. Patient was admitted to Medical Center, where blood work, EKG, and oxygen were given and ordered. After overnight observation, the patient was discharged and informed that he had a severe reaction due to the Pfizer vaccine for Covid-19. As of 1/17/2021, patient still recovering and feeling generally weak and tired without much appetite. | |
| COVID19 VACCINE (COVID19) | Vaccine was administered on 1/12/21 at Memory Care. On 1/15/21 at 12:30 he developed slurred speech at his facility and slumped to his left side. Out of concern for stroke he was sent by ambulance to Hospital. There he was found to have no evidence of stroke on MRI or CT angiogram. He was admitted to the hospital due to fever and elevated inflammatory markers (ferritin, CRP) and transaminases. He was found to have a positive SARS-CoV-2 PCR and IgG. His symptoms resolved the following morning and may have represented a TIA. He had many markers consistent with COVID-19 and his CT pulmonary angiogram did show ground glass opacities but no pulmonary embolism. It was difficult to assess if this was a reinfection with COVID-19, persistent PCR positivity from November, or an adverse event to the vaccine. | |
| COVID19 VACCINE (COVID19) | I received the dose at 1:45 pm on 1/13/2021 at Medical Center. Almost 1 hour post vaccine I started to feel a lump in my throat, as the minutes passed my throat started to feel tighter and fight and flight mode kicked in. It was hard to swallow, my tongue was swelling and I called EMS at 2:40 pm 1/13/2021. EMS noticed hives on my chest and left arm where I got the first dose of Moderna. I received epinephrine IM and IV benadryl in the ambulance. I was sinus tachycardic with heart rate in the 140s oxygen was 99% room air, lungs clear.. I was taken to ER where I was on observation for 2 hours. I was discharged around 5:10 pm on 1/13/2021. On my car ride home around 5:40pm I again started to feel my throat tighten, tongue swell, and heart race. I called EMS again, and was treated with IV benadryl, and epinephrine IM. I was taken to Medical Center where I was given IV solu-medrol and got blood taken and a pregnancy test done. My potassium was 3.1 and I took 2 potassium tablets to supplement. My EKG was normal sinus tach, oxygen 100% room air, blood pressure 140s/90s and got down to 120/80s. I was transported to another Medical Center for overnight observation because first Medical Center was full. | |
| COVID19 VACCINE (COVID19) | “Narrative: Patient with severe aphasia and only able to say “”hey, hey, hey”” or “”uh huh”” or shake his head no as a way to communicate. Patient previously able to ambulate with significant limp and hyperextension of right knee, but mostly wheelchair bound over last several years as he had had a slow and steady decline in overall health and mobility. Patient developed aggressive behavior of shouting “”hey”” and grabbing of groin in 2016. This was worked up with CT scans, labs, referral to urology, neurology, and referrals to psychiatry. The exact etiology of this action was never able to be affirmed, but thought to be more psychiatrically related. It improved significantly with addition of antipsychotics, worsened when antipsychotics were reduced, and improved again with addition of injectable antipsychotic on 12-10-2020.Patient suffered from falls on occasion given his significantly impaired physical mobility. His last documented fall was 8-31-2019. Patient began utilizing wheelchair most of time following that fall. No significant injuries noted in documentation of the falls. In the last 3 months, patient would often refuse medications. He would sometimes indicate that they would cause dizziness, and other times he would simply refuse. We attempted to hide medications in his food/fluid (with wife’s blessing) and when he detected this he would occasionally refuse to eat. Patient previously on DOAC. After pharmacy review in 12/2020 it was recommended to discontinue this as no clear indication to continue use. He was high fall risk and would often refuse this medication as well since 10/2020. Noted to be in NSR on EKGs and decision made to discontinue the DOAC. Patient had no evidence of adverse effects noted after vaccination on December 28th. Patient seen by provider on the morning of his death (1/4/2021) with no noticeable significant change in health condition. Temperature 36.8Con January 4th at 19:45. During routine bedtime cares, patient suddenly collapsed and death was pronounced January 4, 2021 at 20:05. Autopsy was requested from next of kin and no autopsy was granted. Symptoms: & DEATH Treatment:” | |
| COVID19 VACCINE (COVID19) | Narrative: Symptoms: Palpitations & Syncope Treatment: EPINEPHRINE 1 MG ONCE ,EPINEPHRINE 1 MG ONCE ,SODIUM BICARBONATE 50 ML ONCE | |
| COVID19 VACCINE (COVID19) | Ventricular fibrillation- Code blue | |
| COVID19 VACCINE (COVID19) | Severe Right sided chest pain, right sided muscle spasms and difficulty breathing two weeks after vaccine was administered Diagnosis of bilateral pulmonary embolism was made on presentation to ER. No personal or family history of clots in arteries or deep veins or any risk factors in patient. Received heparin drip, pain medications, muscle relaxants inpatient. Pain progressively improved over days. Was discharged after 6 days on admission. Was discharged on oral anticoagulant (Rivaroxaban aka xarelto) | |
| COVID19 VACCINE (COVID19) | Developed dizziness and nausea within 90minutes of vaccine; then developed tingling, and flushing of my skin. Then rapid heart rate and chest tightness by 2.5hrs post vaccine. I went to urgent Care and they thought it was an allergic reaction (BP 182/90, HR 82) and gave me 125mg solumedrol and Benadryl intramuscularly which caused worsened dizziness and a racing heart which caused me to collapse and they gave me a epi pen and called 911. I was transferred to ER and they completed EKG which was normal and monitored vitals for a few hours and I was released. I continue to remain extremely dizzy and nauseated 2days after the vaccine. | |
| COVID19 VACCINE (COVID19) | “2.5 hours after receiving vaccine, I started getting dizzy and vomiting. I vomited 6 or 7 times. I was so dizzy I couldn’t open my eyes without getting sick. We called ER and explained that we thought I was having a reaction to the vaccine, and their response was they had never heard of that type of reaction. I was sick all night, called my primary Dr in the am and they told me to go to ER. We had to call ambulance to take me there because I was so sick and couldn’t open my eyes without having to vomit. Room spun in circles. My arrival at ER, they did MRI, EKG, blood work. My blood pressure was very high, so they connected me to a heart monitor. I had low Magnesium due to vomiting so much. I was treated with Valium and anti-vert medication and fluids. I stayed overnight in hospital- had PT Jan 13th for DX of Vertigo. Then referred to Outpatient PT for Vertigo. Dr’s felt it was a “”coincident”” that I got Vertigo shortly after vaccine, and strongly felt it was not related. . Today is Jan 17th, and I am still dizzy, but feeling better. I have an appointment with my primary Jan 18th and not sure if I should take the 2nd dose. That is the question.” | |
| COVID19 VACCINE (COVID19) | Acute Appendicitis | |
| COVID19 VACCINE (COVID19) | Heart attack death medical test | |
| COVID19 VACCINE (COVID19) | Resident expired 1/17/21 | |
| COVID19 VACCINE (COVID19) | 29yo female patient reports feeling her throat tingling and closing sensation in her throat with a metallic taste and diaphoretic approximately 3 minutes after receiving vaccine. She did not report these sensations until about 15min after injection. EMS assist was immediately called and pt was brought into one of the patient rooms. She was given Epipen injection approx. 20min after injection and EMS arrived to transport patient down to ER within 1-2 minutes after Epipen administered. Patient was monitored in ER and recovered well | |
| COVID19 VACCINE (COVID19) | lymphadenopathy on left side (starting in axillary and progressing to supraclavicular and eventually neck as well. swollen and extremely tender) in addition to normal side effects of chills, fatigue. also was having irregular heart rhythm (reason for hospitalization) do not know if the heart rhythms are related to the vaccine or not, onset was a few days after vaccination (ER 12/30/2020, then another hospital 1/1-1/2). but the hospitalization below is referring to the heart rhythm not the lymphadenopathy. | |
| COVID19 VACCINE (COVID19) | The patient received her first Moderna COVID-19 vaccination on 12/29/2020. However the patient was diagnosed with a positive COVID-19 test on January 4, 2021. Patient complained of nausea, vomiting, back pain, and sharp chest pain. On January 13, the patient presented to the emergency department again with shortness of breath and sharp, stabbing left-sided chest pain radiating to her back and right side. Initial work up ruled out cardiac etiologies. CTA chest demonstrated COVID-19 pneumonia. The patient complained of bilateral lower extremity weakness which had been progressing since her COVID-19 vaccination, per patient report. However, during her hospitalization the patient’s bilateral lower extremity weakness began to accelerate. On the 13th, the patient was able to ambulate to and from the bathroom herself. Then on January 14 the patient required maximum assistance. Neurology was consulted and work up initiated for suspected possible Guillain-BarrT syndrome (GBS) secondary to recent COVID-19 infection. On January 15, 2021, the patient became obtunded and unable to protect airway. She was emergently intubated for acute hypercapnic respiratory failure secondary to GBS. Neurology started GBS treatment with IVIG. Patient also developed NSTEMI and Takotsubo cardiomyopathy. Patient remains critically ill requiring mechanical ventilation. | |
| COVID19 VACCINE (COVID19) | The day after receiving the second vaccination, I began to have mild intermittent abdominal pain2-3/10. The pain gradually increased, became more intense, and more constant. Mild fever and chills started happening, and I took Ibuprofen. By about 4 days after the vaccine, the abdominal pain was severe enough that I had some difficulty walking and I couldn?t sleep at night. Pain was 6-8/10. I went to the ER, and CT scan with IV contrast showed 18 mm appendicitis. I underwent laparoscopic surgery and it was found to be perforated. It was removed. I am currently recovering in the hospital. I received the vaccine as a health care provider at my hospital, specifically I am a practicing pediatrician physician for over 10 years. | |
| COVID19 VACCINE (COVID19) | anaphylactic reaction/anaphylaxis; This is a spontaneous report from a Pfizer Sponsored Program from a contactable pharmacist. A female patient of an unspecified age received first dose of bnt162b2 (Pfizer BioNTech COVID vaccine, lot number: EK4176), via an unspecified route of administration on 09Jan2021 at 0.3 mL, single (standard like 0.3ml by injection once to deltoid, side unknown) to prevent from getting COVID. The patient’s medical history and concomitant medications were not reported. The patient experienced anaphylactic reaction/anaphylaxis on 09Jan2021. Clinical course: The patient got the vaccine while waiting to go into the watch room, to be watched for a few minutes, and she experienced anaphylactic reaction/anaphylaxis, she went down, they gave her an Epinephrine, she didn’t respond to the first dose, a second dose was given in the arm where the vaccine was given, then she was picked up by an ambulance. Agent stated the caller has been on hold for almost an hour. Caller clarifies dose was given in the arm, it occurred on Saturday with the same lot. Saturday and it went away on Saturday, the patient was worried about it coming back, thus why she asked about Epinephrine pen, the patient was taken to the hospital, and given Epinephrine a couple more times, and it resolved eventually, the patient was not admitted, she went to the Emergency Department. It could have required hospitalization but would most likely say life threatening had she not been treated. Reporter seriousness for anaphylaxis is life threatening. The outcome of event was recovered on 09Jan2021. Relatedness of bnt162b2 to reaction anaphylaxis is related for primary source.; Sender’s Comments: A possible causal association between administration of BNT162B2 and the onset anaphylactic reaction/anaphylaxis cannot be excluded, considering the plausible temporal relationship and the known adverse event profile of the suspect product. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | The day following vaccination, the patient experienced nausea/vomiting, shortness of breath, followed by extremity paresthesia, and increased heart rate. The patient went to the emergency room and was assigned an overnight admission for a potential post-vaccination adverse event. Patient was discharged the following day. | |
| COVID19 VACCINE (COVID19) | The day of vaccination, the patient presented to the emergency room with a complaint of dizziness and syncope. Reported that she became dizzy, presyncopal feelings, and had a witnessed syncopal event. The patient stated she does have vasovagal syncopal events after blood draws in the past. The patient was assigned an overnight admission for a potential post-vaccination adverse event, new onset atrial fibrillation, dehydration, and syncope. Patient was discharged the following day. | |
| COVID19 VACCINE (COVID19) | Patient became sick 3 hours after the vaccine and was found deceased 1 day after his vaccination. He passed away in his sleep. | |
| COVID19 VACCINE (COVID19) | nausea and vomiting possible cause of diabetic ketoacidosis and svt | |
| COVID19 VACCINE (COVID19) | 0900 IM Covid 19 vaccine 0905 Sore throat 0920 Dizzy episode followed by headache 0945 Stridor upon deep breath 1000 Facial tingling, top lip and eye swelling 1015 Present to Emergency Services 1040 IV benadryl – Tingling throughout body, stridor worsening, , visible facial swelling 1045 IV Decadron – Throat swelling worsening, chest heaviness, wheezing 1050 IM Epinephrine 1055 Racemic Epi nebulizer treatment 1100 Facial and throat Swelling reducing, breathing easier, 1105 Breathing back to normal 1430 Discharged from Emergency Services with prescription for Dexamethasone 4Mg for 3 days, 2 allegra 2x daily, famotidine 2 x daily | |
| COVID19 VACCINE (COVID19) | Jan 11-vaccination day. On Jan 14, in afternoon had tinnitus and muffled hearing that went away. The next morning Jan15, complete sudden hearing loss on left ear. Tinnitus and muffled hearing that has not went away. | |
| COVID19 VACCINE (COVID19) | Patient experienced abdominal pain on January 12th. She reported having a headache and joint pain as well. Her temperature was 103.5. On January 14th the patient was admitted for acute appendicitis with perforation, localized peritonitis, and gangrene. Patient was discharged on January 16th post appendectomy. | |
| COVID19 VACCINE (COVID19) | Patient received Moderna Covid vaccine on Friday evening 1/8/21. She awoke Saturday am around 2AM with a severe headache. She also developed a fever during the day. She did not seek treatment until Sunday and went to Hospital ED. She was treated and released same day. She went back to hospital on Monday as she felt worse and she was admitted at that time and treated for a bloodstream infection and meningitis. We are not sure it was caused by the vaccine. She consulted her PCP and her PCP feels it may be more coincidental given her medical HX. | |
| COVID19 VACCINE (COVID19) | A few days after the vaccine I began to have a cough about mid morning which changed to SOB. Covid test on 12/27 which was neg. I went to work on 12/28 but woke up 12/29 with a low grade fever and felt like my throat was on fire. so on the 29th I went and got a Strep and flu test which were negative. I was feeling so bad so on the 6th of Jan do I went to the stand alone ER and got a chest xray and CT scan which revealed I did have pnemonia. I went back home but was feeling worse and was taken to and admitted in hospital for 2 days. Initially I was admitted into the COVID unit but never was diagnosed COVID positive so I was moved off the COVID unit. I was given albuterol pills to try to help with the symptoms but didnt work causing me to have to be admitted and was given several doses of steorid and other antibioics and had to use O2 at night because my stats kept falling. | |
| COVID19 VACCINE (COVID19) | Patient reported to the emergency room from fever and shortness of breath the day after receiving the COVID-19 vaccine. Patient was found to have a UTI and possible sepsis. Unknown if truly related to COVID vaccine. | |
| COVID19 VACCINE (COVID19) | Severe fatigue, Headache frontal and temporal, dizziness/vertigo, tinnitus, | |
| COVID19 VACCINE (COVID19) | “Pt is 33 yo female with h/o multiple drug allergies , including allergy to benadryl. She has received first dose of COVID vaccine made by Phfizer at 3:45. She reports about 10 minutes after the vaccination she started feeling tingling in her lips, throat and prickly sensation on her chest and feeling “”off””. Felt dizzy, developed small hives on her chest. She was attended to immediately at the vaccine site and our team was called to white code. Pt was sitting on the floor, alert , breathing comfortably. Her BP was 151/84, HR 90, O2 Sats 100%. Her lungs were clear the whole time, no wheezing, no difficulty swallowing or talking. Patient received 125 mg of IV solumedrol and 20 mg of pepcid in vaccination room, she felt the same, still breathing comfotably, speaking full sentences, hives faiding away. She was transported to Urgent Care clinic on wheelchair. Pt kept her EpiPen by her site the whole time but refused to used it, states she is afraid to use it and wants to hold off or get it in ER if necessary. About 16:30 patient reported her tingling, prickly sensation In her chest is getting worse, developed sensation of lump in her throat, able to swallow and breath without problems, lungs exam clear. Again recommended to give Epipen but patient again refused as she feels very anxious about getting new medicine. She was able to speak full sentences and breathing well, O2 Sats 100% the whole time, she repetitively refused EpiPen. EMS called and patient transported to ER, ER notified. Pt left in stable condition.” | |
| COVID19 VACCINE (COVID19) | On 01/13/2021 at about 11pm I began having pain in both arms and across my chest. Also nausea and vomiting. At midnight I went to the Emergency room and was diagnosed with a heart attack, underwent emergency catheterization and stent placement. I had complete occlusion of the right coronary artery | |
| COVID19 VACCINE (COVID19) | Patient is 39-year-old male with no significant past medical history who works at long-term care facility. He received COVID-19 vaccine on 1/13/2021 and immediately developed numbness and tingling in the area of injection which was the left shoulder. Over the next 15 to 30 minutes numbness and tingling expanded to the left side of his body including face arm and leg and the left side of his trunk. He began to feel foggy and had some slurred speech. He presented to the Emergency Room and tests were performed to rule out a stroke. The patient denies any other symptoms such as fever chills myalgias. He did not have any weakness of the extremities. He did not have any speech disturbances or visual disturbances. This morning on examination he states his symptoms are much better. He still has some residual numbness in his leg and arm. Facial numbness is almost all gone. As of 1/18/2021, residual numbness and tingling is only in his left ring and pinky finger. | |
| COVID19 VACCINE (COVID19) | “Patient with PMH of depression and GERD who presented 1/8 with constipation, abdominal discomfort and worsening dyspnea. Symptoms began around 12/29. COVID vaccine 12/19. Previously quite active, marathon runner, gained some weight over last couple years but was still in good enough shape to complete 10K in New Orleans in early February. In late February, had a flu-like illness, as did one of his friends from church. 2020 was hard on him – weight gain, decreased activity, stress, overall deconditioning. No issues apart from sore arm after COVID vaccine 12/19 but then starting getting abdominal fullness/discomfort around 12/29, which steadily worsened, also develop worsening dyspnea on slight exertion. No known sick contacts.. Work-up notable for pericardial effusion, pleural effusions. Echo with severe diffuse LV hypokinesis, concern raised for myocarditis. COVID PCR negative, serology negative. RVP negative. . Concern raised that COVID vaccine may have played a role in myocarditis. He was found to have the following conditions Acute heart failure with reduced EF NYHA FC II, non-ischemic cardiomyopathy. Myocarditis appears subacute per MRI hypertension obesity small pericardial effusion- asysmptomatic no pericarditis suspected obstructive sleep apnea. .Started on the following medications. Continue Carvedilol 12.5mg BID, Farxiga 5mg daily, Digoxin 0.125mg daily, Entresto 97-103mg BID, and Spironolactone 25mg daily. Per MD note. While it remains uncertain, team is doubtful COVID vaccine played a role in his cardiac issues. Given the MRI findings are not acute, more likely that the cardiac insult occurred weeks to months ago – potentially in the setting of the February 2020 illness. Perhaps his “”deconditioning”” in 2020 was related to worsening cardiac function. Nevertheless, will hold on 2nd COVID vaccine dose given absence of a clear explanation for his myocarditis. conversation with team will continue to determine if candidate for second covid vaccine. If consensus is that myocarditis pre-dated vaccine, might be able to proceed with dose 2 of vaccine.” | |
| COVID19 VACCINE (COVID19) | “PATIENT RECEIVED VACCINE 1/7/21 AT 1000. THE NEXT DAY, PATIENT PRESENTS TO ER ON 1/13/21 WITH COMPLAINTS OF PALPITATIONS AND DIZZINESS OCCURING SINCE HER FIRST DOSE OF VACCINE. “”The patient states she has had these episodes for the last 6 days and they started when she got her COVID vaccine. The patient states she has had numerous episodes over the last 6 days of this that lasts between 2 and 4 hours. The patient can feel her heart fluttering and she gets a little dizzy with it. The patient has not had any nausea, shortness of breath or chest pain with these episodes however. The patient has not had any prior episodes of this. The patient takes no regular medications apart from vitamins.”” PATIENT WAS DIAGNOSED WITH NEW FOUND A. FIB WITH RVR. ADMITTED TO HOSPITAL FOR OBSERVATION. PATIENT RETURNED TO NORMAL SINUS RHYTHM THE NEXT DAY AFTER INITIATING DILTIAZEM AND ELIQUIS.” | |
| COVID19 VACCINE (COVID19) | EDD July 4, 2021 January 15th baby no longer had a heartbeat after two previous visits confirming heartbeat and EDD. | |
| COVID19 VACCINE (COVID19) | DVT in right leg 4 days after injection, severe pain in thigh/calf, difficulty walking Placed on Xarelto 15mg 2X daily for 21 days and then 20mg daily for 9 days. Next Doctor visit is 1/26/2021 at 9:00am Next scheduled Covid 19 vaccine is scheduled for 2/5/2021 at 7:15am | |
| COVID19 VACCINE (COVID19) | After I received the vaccine went home rested temp went up one time 99.0. The next day woke up heart was pounding check my BP 150/80 laid back down. I woke up around 5pm my BP had elevated to 160 (don’t remember diastolic reading) pulse rate normal limits. I informed my spouse of what was happening called 911. At the hospital checked vitals, EKG, CT Scan, Chest X-ray, Blood work and Potassium was low took 2 Potassium pills. I went back home on 12/31 returned to work did fine that day. On New Years day everything started back again discomfort went back to ER. I felt like force in my chest the doctor collected my information and I was placed on a low dose BP medication until I return to my PCP, cardiologist(stress test). I followed up with PCP via Telehealth about my BP concerns, my dosage was increased to 25 mg and due to my symptoms I was placed on leave from work 2 wks. I also have a appointment scheduled with Neurologist. | |
| COVID19 VACCINE (COVID19) | Daughter call in for VAERS report to file for father whom committed suicide 1/16/2021 in the AM after reportable ae of COVID 19 vaccine administered 1/14/2021. Patient sought care twice at ER; first visit by ambulance around 5PM and Friday 1/15/2021 Medical Center: Emergency Room. 1st Discharge summary diagnosis: adverse reaction to COVID shot; 2nd Discharge summary diagnosis: adverse reaction to COVID shot, fever, Panic Disorder– ER. Medical Center Discharge summary diagnosis: Adverse reaction to the vaccine, acute anxiety. Reportable patient symptoms at, 1st visit : fever, shaking stomach cramps, breathing issues. Medical Center — No fever, confusion and dementia type, patient would not stay in patient bed; patient would get up and sit down again repeatedly, agitated and anxious. Attempted to urinated hospital bed. Patient committed suicide in home. | |
| COVID19 VACCINE (COVID19) | Anaphylaxis (urticaria, tongue swelling, subjective difficulty breathing) starting approx. 24hrs first moderna dose. No prior episodes of anaphylaxis/allergic rxn. Treated with Benadryl 100mg PO (prior to arrival, pt administered), famotidine 20mg IV, Epinepherine 0.3mg IM. Monitored in ED, complete resolution of symptoms, discharged home. | |
| COVID19 VACCINE (COVID19) | Weakness, Low O2, death. Positive for COVID on 1/12/21, dies on 1/16/21 | |
| COVID19 VACCINE (COVID19) | Patient presented to ED with complaint of chest pain, radiating down left arm, not relieved with Tums. Symptoms started at 0530 1/12/2020. Patient presented to ED b/c of strong family history of CAD, with father having MI in his 50s. | |
| COVID19 VACCINE (COVID19) | On 1/17/2021 at 4:35 am resident found apneic and pulseless, at 4:40am death confirmed | |
| COVID19 VACCINE (COVID19) | I was vaccinated at 3:30pm . At 5:27pm while driving home i felt a cold sensation in the back of my neck and back of my throat which began spreading to the back of my head . My heart felt as if I was startled by something. I looked at my smart watch and my heart rate was 145. I began trembling and having abdominal cramping . The back of my head felt like I had swelling or collection of fluid. I opened my windows and began taking slow deep breaths to bring down my heart rate . It took quite a while to get it below 100. I felt as if I was going to pass out. After deep breathing for what felt like atleasr 15 to 20 minutes , my pulse came down and I closed my windows . As soon as my body warmed back up in the car , the symptoms returned and my heart rate went back up to 130s , 140s . I had to keep my windows down and deep breathe the entire way home which took an hour . My body was trembling. When I got home I felt as if I was too week to get out of the car . I still felt that startled feeling in my heart and was afraid of what could happen next . My lips and face were swollen. My lips were also slightly itchy. I called 911 for help . By the time they arrived my vital signs had stabilized but I still had swelling in my face and lips . My EKG , vital signs and oxygen levels checked out normal so I did not go to the ER. That night I took benadryl and Tylenol. Day 2 post vaccine the collection of fluid or swelling in the back of my head had now spread to the top . That night I had the feeling that my throat was swelling do I took benadryl and Tylenol and my face and lips were still slightly swollen . Day 3 post vaccine I woke up with slightly blurry vision. The swelling in my head now feels like it has encompassed my entire head and have a slight headache. I went to the urgent care requesting an MRI of the head and an epi pen . I was given Medrol dose pack , an RX for epi pen for emergencies and advised to continue benadryl and Tylenol. Day 4 post vaccine, slight headache continues. Slightly blurry vision | |
| COVID19 VACCINE (COVID19) | 12/29/2020 Vaccination. Within minutes blurry vision, dizzy, tx to stretcher. EXTREME HA, coughing, sensitivity to light. EPI pen in ER. I fell asleep. Woke up, don’t remember much. Admitted to hospital. Chest pains started within 2 hours, SOB, HA. Admitted for 2 nights, 3 days. Discharged home. Still having SOB and referred to Pulmonologist. Waiting on appt. *did NOT have problems before this vaccine. I was fine. Now i am completely different person, I have to monitor my walking, etc. | |
| COVID19 VACCINE (COVID19) | “80YO male who htn, cva, epilepsy, ckd, cerebral avm s/p repair, cad s/p cab, cva (left sided hemiplegia) , hx of prostate cancer recent admission for pna on abx presents to ED on 1/11 with dizziness, hypoxia. CT with Bilateral PE “”Large bilateral pulmonary artery emboli in the right and left main pulmonary artery extending into the right and left main pulmonary artery branches bilaterally. Findings are associated with right-sided heart strain.”” “”Patchy alveolar airspace disease within the lungs highly suspicious for COVID pneumonia”” Covid negative. Patients wife recovered from Covid-19 infection within last month. Patent thus far has tested negative. Doppler lower extremity revealed Acute occlusive vein thrombosis of the entire course of the gastrocnemius vein and soleal vein. Patient received covid vaccine on 1/4/21. Patient has several risk factors for clot – age, previous CVA, hx of prostate cancer. Also had positive covid exposure though tested negative” | |
| COVID19 VACCINE (COVID19) | Resident was seen by MD on 1/11/2021 due to increasing in edema and shortness of breath. Lasix 40 mg STAT given. New orders to get a STAT CBC, CMP, and BNP. Resident has been dependent on Oxygen since his diagnosis of COVID-19 on 11/23/2020. Labs were abnormal. Continued on the lasix 40 mgs. Resident remained short of breath with exertion and on oxygen. He was assisted to the toilet on 1/15/2021 in the morning where he subsequently passed away. | |
| COVID19 VACCINE (COVID19) | Death (death certificate: uncertain type of death); evening vomiting; This is a spontaneous report from a non-contactable physician the Regulatory Authority. This is a report received from the Regulatory Authority. Regulatory authority report number was DE-PEI-CADRPEI-2021011672. A 79-year-old female patient received BNT162B2 (COMIRNATY; Lot Number: EJ6796), intramuscular on 30Dec2020 as a single dose for COVID-19 immunization. Medical history included especially autoimmune encephalopathy of paraneoplastic origin and suspected urothelial carcinoma of the bladder. The patient’s concomitant medications were not reported. The patient previously received the influenza vaccine (MANUFACTURER UNKNOWN) on 03Dec2020 for immunization and was tolerated. On 31Dec2020 (also reported as 30Dec2020), the patient developed evening vomiting which was dark in color and most likely food related. On 31Dec2020 at 04:35, the patient died. It was not reported if an autopsy was performed. The clinical outcome of vomiting was reported as fatal; however, the cause of death was reported as unknown cause of death. The vomiting and unknown cause of death were reported as medially significant and fatal. No follow-up attempts possible. No further information expected.; Reported Cause(s) of Death: Unknown cause of death | |
| COVID19 VACCINE (COVID19) | difficult breathing; Pain on the chest; Not being able to move my upper neither lower body; itchiness all over my body; unconscious; Headaches; major anxiety; depression; very light-headed; weak; almost faint; This is a spontaneous report from a contactable Other HCP (patient). A 28-years-old female patient received first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE) on 08Jan2021 12:00 at single dose on Left arm for COVID-19 immunisation. Facility type vaccine was Hospital. Medical history was none. The patient’s concomitant medications were not reported. No other vaccine in four weeks. The patient experienced Not being able to move my upper neither lower body, Headaches, major anxiety, and depression, unconscious, very light-headed, weak, almost faint, Pain on the chest, difficult breathing, itchiness all over my body on 08Jan2021. AEs resulted in Emergency room/department or urgent care, Hospitalization. Received injected fluids as treatment. The outcome was Not Recovered. No COVID prior vaccination. No COVID tested post vaccination. Information on the lot/ batch number has been requested.; Sender’s Comments: Based on a close temporal association, a causal relationship between reported events and BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE) cannot be excluded. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | SOB; leukocytosis; acute onset of fever (101.2); CXR = bilateral vascular congestion; This is a spontaneous report from a contactable consumer. An elderly NH resident patient of unspecified age and gender received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE) on an unspecified date, at single dose, for COVID-19 immunization. Medical history and concomitant medications were not reported. On an unspecified date the patient experienced shortness of breath (SOB), leukocytosis (17,800), acute onset of fever (101.2), bilateral vascular congestion. The patient was admitted with acute onset of fever (101.2), leukocytosis (17,800, no eosinophilia) and SOB, all beginning 3-4 hours after BNT162B2 administration. CXR showed bilateral vascular congestion. No facial or SQ edema. No evidence of myocardial damage or CHF based on BNP and troponin, respectively. The patient was treated with brief (3 days) pulse of methylprednisolone sodium succinate (SOLUMEDROL) with improvement. Chalking it up to acute capillary leak syndrome 2^2 vaccine. The final events outcome was unknown. Information on lot/batch number has been requested. | |
| COVID19 VACCINE (COVID19) | Patient presented to our Emergency Department via EMS in full code status; asystole. Patient expired. Per nursing, husband stated patient awoke this AM and reported pain in back between shoulders and in bilateral shoulders. Patient then went unresponsive and husband called EMS. | |
| COVID19 VACCINE (COVID19) | 1/11/21 at 8:57 Resident with fever and at 11 am saturation down to 83 O2 to 10 liters. Resident continued to decline until CTB on 1/14/2021 at 1325 | |
| COVID19 VACCINE (COVID19) | I was diagnosed with COVID-19 on 12/7/2020. My course of symptoms lasted 16 days, meaning I started feeling healthy again on 12/23/20. I am a pharmacist with a healthcare system and they have been offering the Pfizer/Biontech COVID-19 vaccine for essential associates. I received by first dose of vaccine on 12/31/20. On 1/1/20 I woke up with very noticeable muscle aches and fairly profound lethargy, which last 18-20 hours. I was not able to do much on 1/1/21 because of the way I was feeling. I’m not sure if this reaction is normal for patients who receive their COVID-19 vaccine close to their illness/infection with COVID-19, which is why I’m reporting this to the FDA. | |
| COVID19 VACCINE (COVID19) | Heart rate slowed significantly down to 32bpm Tightness in chest, trouble breathing Elevated blood pressure | |
| COVID19 VACCINE (COVID19) | injection site became red & swollen, the size of a softball. Employee started having seizure like symptoms on 1/14/21 and was admitted to hospital. DC from hospital on 1/16/21 and on 1/17/21 started having seizures again and readmitted back to hospital. Employee has no history of seizures. | |
| COVID19 VACCINE (COVID19) | I received the Pfizer vaccine on 12/21/20 without adverse events other than soreness in deltoid. On 12/31/20 I began to notice pain, redness and swelling in second toe on R foot from base of toe to base of nail. I initially thought it was gout and self medicated with ibuprofen 400 mg BID x 1 day on 01/01/21 with some improvement. I did not take anything on 01/02/21 and woke up on 01/03/21 with return of swelling, redness and pain. I took another 400 mg of ibuprofen and it felt better but after examining the toe, I questioned whether I had developed pernio (COVID toe). | |
| COVID19 VACCINE (COVID19) | patient began with vomiting and diarrhea the day after administration, leading to bowel and urine incontinence. patient was hospitalized on 01/16/20 with sepsis. no origin discovered yet. still waiting on blood/urine/stool cultures. | |
| COVID19 VACCINE (COVID19) | Patient was living in a nursing home with positive cases when administered. His age and chronic condition was such that he did not have time after the vaccination to avoid exposure or develop immunity. | |
| COVID19 VACCINE (COVID19) | Resident had seizure like activity that was about 30 minutes in duration where her upper/lower extremities were shaking uncontrollably. Resident had to be admitted into hospital for observation. | |
| COVID19 VACCINE (COVID19) | blistering rash – bullous pemphigoid, steroids, admitted to hospital pending further evaluation. | |
| COVID19 VACCINE (COVID19) | 1day after vaccine,developed severe headache & later blister in head officially Shingle . Then decreased platelet count fatally to 29(ITP).now hospitalized getting treatment. | |
| COVID19 VACCINE (COVID19) | Was feeling anxious right after vaccine given. Laid in cot for a short time, then stated her throat felt like it was closing. | |
| COVID19 VACCINE (COVID19) | Severe rash. Platelets drop to almost needing transfusion | |
| COVID19 VACCINE (COVID19) | 1252 Resident rang with complaints of chest pain and shortness of breath. BP 126/70, Temp 97.5, pulse 72, resp. 20, O2 sats on room air 90%. While awaiting transport complained of increasing shortness of breath. Resident transported to Community Hospital via Ambulance with 3L O2 . Resident placed on ventilator and transported to Medical Center | |
| COVID19 VACCINE (COVID19) | resident expired; This is a spontaneous report from a contactable healthcare professional. An 82-year-old male patient received the first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 MRNA VACCINE; Lot number: EL0140), intramuscular in the left arm on 05Jan2021 15:00 at a single dose for COVID-19 immunization. Medical history included metabolic encephalopathy from, failure to thrive (FTT), diabetes mellitus (DM) 2 , chronic obstructive pulmonary disease (COPD), arthritis, weakness, hyperlipidemia, chronic kidney disease (CKD), dementia. Known allergies was none. The patient took unspecified concomitant medication. On 11Jan2021, the resident expired. The patient underwent lab tests and procedures which included nasal swab: negative on 09Jan2021. There was no treatment given for the event. The patient died on 11Jan2021. An autopsy was not performed.; Sender’s Comments: Lacking information on the cause of patient’s demise, the Company cannot completely exclude a causal relationship between COVID 19 vaccine, BNT162B2, and patient’s death of unknown cause, as a cautionary measure and for reporting purposes. The patient’s pre-existing medical condition of metabolic encephalopathy from, failure to thrive (FTT), diabetes mellitus (DM) 2 , chronic obstructive pulmonary disease (COPD), arthritis, weakness, hyperlipidemia, chronic kidney disease (CKD), dementia may have provided the contribution to the event in this 82-year-old male patient. The impacts of this report on the benefit/risk profile of the product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate.; Reported Cause(s) of Death: resident expired | |
| COVID19 VACCINE (COVID19) | 1) Skin rash over 80% of my body including, face and lips; started to change my voice sound and started to compromise my airways. 2) Uncontrollable shakes, but not sure if this was related to Covid-19 itself. Was given steroids via injection into my blood stream, within minutes the shakes stopped and within 2 hours the rash was gone. | |
| COVID19 VACCINE (COVID19) | 12/22 Vaccine 12/23 ER. Admitted to hospital for 7 days. Dr stated was due to fluid. Radiology stated no fluid present. Couldn’t breath. Pulmonologist put on 2nd inhaler. Coughing a lot. Not able to walk more then 6 steps without stopping to catch breath. *Has had 2nd vaccination on 1/13/2020 | |
| COVID19 VACCINE (COVID19) | 1/12 vaccination 1/13 swelling and pain at injection site. Admitted for 4 days. | |
| COVID19 VACCINE (COVID19) | patient suddenly developed pneumonia 7 days after vaccination and died the evening of developing pneumonia | |
| COVID19 VACCINE (COVID19) | Couple hours after the vaccine, experienced elevated temperature, difficulty taking full deep breathes (attributed to fatigue). Temperature improved but fatigue and shortness of breathe continued until 1/16/21. a bilater arm rash occurred and employee went to the Emergency Department for an assessment. At this time, she was having chest tightness, shortness of breathe. Lab – troponins elevated. Other labs – within normal limits. Admitted to hospital for observation and discharged next day. Today, during cardiac follow-up appt, was having decreased oxygen saturation levels, increased pulse rate. Admitted again today for obsevation with cncern of acute endocarditis. | |
| COVID19 VACCINE (COVID19) | Death |
| COVID19 VACCINE (COVID19) | 1 hour post injection patient returned with redness and borderline hives in her left arm, chest, neck and face. She complained of feeling very hot and with mental confusion. We administered 50 mg of diphenhydramine, and 15 minutes later sent her to the ED. At the ED they diagnosed her with a minor anaphylaxis reaction, gave her methylprednisolone and epinephrine. | |
|---|---|---|
| COVID19 VACCINE (COVID19) | “Individual felt fine except a sore arm until 1/5/2021 legs became weak and dizziness and light headed and did t”” feel well”” and passed out. had general muscle weakness and went to ER. She was given fluid for possible dehydration and went home and weakness increase and went back to ER and was admitted to hospital for testing.” | |
| COVID19 VACCINE (COVID19) | Individual had no side effect except sore arm for 5 days then on 1/5/2021 became weak and dizzy and passed out. had not felt well in am. she had continued weakness and extreme leg weakness and taken to ER for evaluation. She states got IV fluids and sent home but weakness increase and went back to ER on 1/5/2021 and admitted to hospital. | |
| COVID19 VACCINE (COVID19) | patient started to decline 1/10/2021, patient seen at facility by medical professional – patient deceased 1/13/2021 | |
| COVID19 VACCINE (COVID19) | REPORTING ONLY AS RESIDENT EXPIRED ON 1/17/2021 3 DAYS AFTER. S/S HYPOXIA/CONGESTED LUNG SOUNDS | |
| COVID19 VACCINE (COVID19) | ventricular fibrillation cardiac arrest. Witnessed collapse. Bystander CPR performed. Paramedics performed ACLS with defibrillation x 6 before ROSC | |
| COVID19 VACCINE (COVID19) | PVCs with compensatory pauses, postural orthostatic hypotension associated with chest tightness, shortness of breath, dizziness and blurry vision | |
| COVID19 VACCINE (COVID19) | The day following the vaccine, the patient complained of throat issues and anxiety. This was not new… however . That evening he reported difficulty breathing and was placed on oxygen; a COVID test was performed and was negative. On 12/30/2020, patient complained of sternal pressure and was transferred to the hospital. The patient died 12/31/2020 and records obtained from the hospital indicated the patient died from a massive myocardial infarction. | |
| COVID19 VACCINE (COVID19) | Seizure | |
| COVID19 VACCINE (COVID19) | WITHIN 30 SECONDS OF RECEIVING VACCINE PATIENT STATED THAT SHE DID NOT FEEL WELL. HER FACE BECAME FLUSHED. HER LIPS BECAME NUMB AND HER TONGUE AND THROAT STARTED SWELLING. AN EPIPEN WAS ADMINISTERED AND 911 CALLED. AFTER THE EPIPEN SYMPTOMS BEGAN TO RESOLVE. EMS CHECKED HER OUT AND SHE REFUSED TRANSPORT. | |
| COVID19 VACCINE (COVID19) | 8 hours after vaccine severe injection site pain/swelling, severe body aches, 101.0 temp. 16 hours after vaccine woke up from sleeping with flushed skin, facial swelling, and throat swelling. I immediately took 100mg of Benadryl and went to hospital emergency room. Approximately 30-40 minutes later symptoms started to lessen. Once at the ER, at the same time symptoms began to resolve, I was given PO Solumedrol and Pepcid. I was monitored and then discharged with RX for prednisone, and EPIPEN (to use if needed). No other issues with allergic reaction. Mild injection site soreness, mild body aches, 99.3 temp persist at 36 hours post injection. | |
| COVID19 VACCINE (COVID19) | Received Moderna COVID vaccine 12/31/20, 3 days later noticed generalized joint pain all over. Day 4 noticed both knees were red and tender and could palpate pockets of fluid. Had bilateral ankle and foot pain, right ankle swollen. Overnight 1/5-1/6/21 was in severe pain and unable to sleep, very difficult to walk on ankles and feet, stairs were very painful. Motrin relieved symptoms to where able to walk more comfortably but generalized achiness and tenderness to ankles and knees remain. | |
| COVID19 VACCINE (COVID19) | Anaphylaxis after Covid 19 vaccine #1. Pfizer Lot # EH9899 | |
| COVID19 VACCINE (COVID19) | 71 year old woman at rehabilitation center for physical therapy with history of cirrhosis of the liver, asthma, and heart condition was tested for COVID-19 on 01/07/21, received 1st dose of Pfizer COVID-19 vaccine on 01/08/21, positive test result for COVID-19 received on 01/09/21. She was sent to the hospital and admitted on 01/12/21 after O2 was 70% and was in a confused state. Patient passed away on 01/17/21. | |
| COVID19 VACCINE (COVID19) | Received shot Wednesday night, developed arm soreness and mild flu like symptoms on left side of my body and facial paresthesias on the left side of my face. Twelve hours later, after waking up those same symptoms were only on the right side of my body. Friday morning, mostly normal physically just with some overall fatigue. Friday afternoon I started to get hives on my chest and overnight into Saturday they were on my lower back, sides, and legs. I took 50 mg of Benadryl every 6-12 hours until Monday mid-day when Benadryl was not helping reduce the hives and so I had full body hives. I did try an drugstore cortisone cream which did not help. Sought treatment at an urgent care as I was feeling anxious and could not control the itching. I and was diagnosed with likely allergic reaction to the covid-19 vaccine. | |
| COVID19 VACCINE (COVID19) | Hospitalized for stroke on 31Dec two days after vaccine.; sudden loss of hearing to right ear; dizzy and lightheaded/severe dizziness/felt like fainting; difficulty breathing; vomiting; This is a spontaneous report from a contactable nurse (patient). This 43-year-old female patient received the first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE), (Lot number: EL1285) via intramuscular route on 29Dec2020 14:30 at single dose on the left arm for COVID-19 immunization. Medical history included anxiety. No known allergies. Concomitant medications were not reported. Patient was not pregnant. Facility type vaccine was Nursing Home/Senior Living Facility. No other vaccine received in four weeks. Patient hospitalized for stroke on 31Dec2020 two days after vaccine (Days of hospitalization: 4). Day of vaccine-6 hours after patient had dizzy and lightheaded for about 45 min then went away. On 31Dec2020 at 19:15 had sudden loss of hearing to right ear and severe dizziness, difficulty breathing, vomiting, and felt like fainting. Paramedics were called-sent to ER, had CTA which showed partial blockage and received TPA. Treatment included TPA, fluids, medications, hospital stay, outpatient follow ups, physical therapy. Patient was not diagnosed with COVID prior to vaccination. Patient has been tested for COVID post vaccination. The patient underwent lab tests and procedures which included Nasal Swab: Negative and Pixel: Negative on 08Jan2021. Outcome of the events was recovering.; Sender’s Comments: The Company cannot completely exclude the possible causality between the reported events including stroke, dizzy/lightheaded/felt like fainting, difficulty breathing, vomiting, and sudden loss of hearing to right, and the administration of the COVID-19 vaccine, BNT162B2. More information regarding the patient’s underlying medical conditions, relevant lab tests would be helpful for the Company to make a more meaningful causality assessment. | |
| COVID19 VACCINE (COVID19) | Developed chest tightness around right side of chest into back and SOB 50.5 hours after vaccination. Went to local ER and found to have a right lower lobe pulmonary embolism. Treated with Xarelto and sent home with outpatient follow up. | |
| COVID19 VACCINE (COVID19) | 9th: cold (?fever?), restless, body aches (especially headache, neck pain, bilateral knee pain), nausea, vomiting 10th: profound fatigue, hives, intermittent vertigo 11-17th: vertigo, mild headache and neck pain, nausea, vomiting 18th-current: vertigo, nausea, vomiting *Hospitalized from 17-18th, diagnosed with vestibular neuritis secondary to the vaccine | |
| COVID19 VACCINE (COVID19) | 1/16/21, Covid vaccine injection at 12:09 PM Minute 1: dizzy and light headed (like drinking a beer on an empty stomach) Minute 10: Nausea Minutes 23-25: Neck tightness (like doing unsupported crunches and holding my head up) Minute 27: Inability to swallow and inability to speak EMS on site administered EpiPen auto-injection to left thigh, immediate improvement in symptoms Transport to hospital via ambulance Hospital monitored me for several hours and discharged same day | |
| COVID19 VACCINE (COVID19) | itching, hives, short of breath, numbness and tingling to lips with hives to bottom. headache. | |
| COVID19 VACCINE (COVID19) | On 1/13/2021, resident had sudden emesis. Immediately following emesis he was noted without a pulse and pronounced deceased. No acute symptoms noted prior to this episode. Resident does have a significant cardiac history. | |
| COVID19 VACCINE (COVID19) | Started with severe chills, body aches and feverish. The. Slight leg pain which worsened with time , swelling on the right leg calf, warm to touch and difficulty breathing. Got hospitalized on 1/16 21 with multiple clots in my right leg and clot in the lung. Still in the hospital now. | |
| COVID19 VACCINE (COVID19) | She had the first dose of Pfizer vaccine at the Campus on Friday 1/15 at 4:30 pm. After the vaccine, she had no new symptoms or signs of vaccine reaction and MD friend reports that he checked her pulse which was not elevated from baseline. On 1/16, she awakened and continued to feel at her recent baseline. However, in the early afternoon, she complained of headache, nausea/epigastric pain, and chest heaviness. These apparently were not unusual symptoms for her to feel intermittently. Per her niece, who has a home O2 sat device, her 02 sat that morning was 97 with a HR of 87 irregularly irregular. She was afebrile. (continue on page 2) | |
| COVID19 VACCINE (COVID19) | Aphasia, ,right-sided weakness and garbled speech | |
| COVID19 VACCINE (COVID19) | “Had severe body aches m, fever, headache, progressed into dizziness and “”foggy memory””, started to have some chest pain. Felt as if I was intoxicated, lasted the whole day. Woke up the next morning and still felt “”out of it”” and weak but thought it would get better, went to work (im a nurse). Started having continous vomiting, shortness of breath and chest pain at work. As well as severe tremors. I was taken to the hospital, given fluids and my QT was prolonged with my heart which i have never had before . Was given iv magnesium and waited for my heart rhythm to improve. Was told not to take anymore of my prescribed medications or nausea medications and follow up with my pcp the next day. Im still feeling horrible, nausea, body aches, low grade fever and I am 72 hours out. Now I have huge hospital bill to pay, can’t work currently because I still feel bad and my heart has a weird rhythm. Hoping this helps as if this was what I was expecting I would have never got it.” | |
| COVID19 VACCINE (COVID19) | Pt complained at ED of Headache, Nausea, SOB, felt like had been running. Pt in AFIB started on cardizem admitted to hospital 1/15 discharged 1/17 | |
| COVID19 VACCINE (COVID19) | Patient tested positive for COVID 19 on Jan 5th 2021 Patient was admitted to the hospital January 9th for pneumonia due to COVID | |
| COVID19 VACCINE (COVID19) | Acute liver injury requiring transplant evaluation and acute kidney injury | |
| COVID19 VACCINE (COVID19) | Anaphylaxis after Covid 19 vaccine #1. Pfizer Lot # EH9899 | |
| COVID19 VACCINE (COVID19) | Two days after her shot, she was sitting down working on her computer paying bills. She became nauseas and dizzy and then fainted. She hit the tile floor. | |
| COVID19 VACCINE (COVID19) | Lot number for the first dose was EK5730. After receiving the 2nd dose on the 01/11/2021 went to lunch and in the evening started feeling nausea and was throwing and abdominal pain. Went to work sick Tuesday and Wednesday low grade fever, nausea and abdominal pain, wasn’t eating was to sick to eat. Thursday early morning hours the pain was unbearable so she went to the ER and the cat scan. It was determined that she had appendicitis and they removed my appendix same day. Feels so much better now. | |
| COVID19 VACCINE (COVID19) | “After receiving Moderna vaccine, pt became increasingly tired, withdrawn, and confused, refusing to walk at home. He has begun to have mild memory changes after suspected COVID illness (covid testing negative) in November, but daughter of patient, with whom he lives, states that his memory and orientation now significantly changed- he seems to have forgotten the last “”3 years”” of memory. Presented to ER 1/16/21 as she checked his O2 and found him to be hypoxic in 60s. He is being treated for possible CAP with underlying perviously undiagnosed ILD vs post-covid lung changes (per pulmonology), and his energy and ability to walk have returned but memory is significantly impaired, confabulating and oriented only to self despite good oxygenation on 5L O2 by NC.” | |
| COVID19 VACCINE (COVID19) | About 22 hours after the shot, I had a mini stroke that required going to the emergency room by ambulance. I was transported that evening to the stroke division that same evening for further evaluation, tests and care. I have never had a mini stroke before this.The doctors said it may have been from the vaccination, or it may not have been precipitated by it. They said they don’t have enough information on the Moderna vaccine to make that call. | |
| COVID19 VACCINE (COVID19) | Patient was vaccinated in right arm. Within 5 to 10 seconds after vaccination, patient started clinching his hands tightly and became unresponsive. Patient was lowered to the floor and did not exhibit a pulse. CPR was initiated and 911 was called. An AED was used and healthcare professionals onsite continued compressions until the paramedics arrived. | |
| COVID19 VACCINE (COVID19) | Death | |
| COVID19 VACCINE (COVID19) | Received vaccination on 12/30/2020, had positive Covid test result on 1/6/2021, was hospitalized with respiratory issues on 1/11/2021, and discharged home from the hospital on 1/18/2021 | |
| COVID19 VACCINE (COVID19) | 12:52 PM resident rang her call bell with complaints of chest pain and shortnes of breath. BP 126/70, Temp 97.5, Pulse 72, Resp. 20, O@ stats on room air 90%. resident requesting transport to ER. EMS called at their arrival resident had increasing SOB o2 on at 3L Nasal Canula. Transported to hospital. Resident placed on ventilator and tranported to different hospital. | |
| COVID19 VACCINE (COVID19) | “Felt tachycardia immediately, thought she was anxious. After 35-45 minutes she felt like she was having a hard time swallowing which progressed to tongue swelling, all taste buds popped up and sore, hives on face & neck, reddened face. Itchy neck and face. Took double dose of Atarax and went to bed. Felt extremely fatigued unsure if double dose of Atarax. Woke with swelling all over body. Woke up feeling heaviness as if she had “”sumo wrestler”” on her body. 24 hours post vaccine heaviness started to lift but felt as if she had a vise on her lungs. Continuing to take Atarax every 6 hours per MD order.” | |
| COVID19 VACCINE (COVID19) | Resident received vaccination on January 15, 2021. She was found unresponsive with shallow respirations on the morning of January 16, 2021 and was sent to ER via ambulance. The resident was admitted to medical center ICU where she passed away later that day. | |
| COVID19 VACCINE (COVID19) | resident had a pressure ulcer to RT hip, was getting treatment on. Was scheduled to have wound debrided and wound vac applied on 1-19-2021. Appetite was poor, not wanting to get out of bed, and decline in alertness. Passed away on 1-16-2021 | |
| COVID19 VACCINE (COVID19) | patient received vaccine 12/29. Unexpected death 1/5. | |
| COVID19 VACCINE (COVID19) | Patient had received second Pfizer vaccination after no reported issues with the first dose. Patient was observed post vaccination without incident and released. Patient developed wheezing and attempted to treat with her albuterol inhaler but did not improve. patient presented to the ED approximately 2 hours after vaccination and was admitted for respiratory failure and placed on BiPAP for a brief period of time. patient has known history of Asthma Exacerbation requiring hospitalization and intubations. D/C diagnosis Asthma Exacerbation | |
| COVID19 VACCINE (COVID19) | Systemic: Anaphylaxis-Severe; symptoms lasted 1 day | |
| COVID19 VACCINE (COVID19) | Systemic: Anaphylaxis-Severe, Systemic: Seizure-Severe | |
| COVID19 VACCINE (COVID19) | Temp of 104.5, hospitalization | |
| COVID19 VACCINE (COVID19) | COVID 19 Vaccination administered by pharmacy staff. No adverse effect at the present time. Staff will continue to observe adverse reaction. Will continue to monitor. Patient at start of shift awake in the bed. Pt at 3am was on the commode leaned to the side. Patient body still warm to touch no pulse. Called for assistance Asap. Cpr started promptly. Cpr given patient on floor 911 arrived at the scene at 3:10am Cpr rotated Between Nursing and EMT on Scene. Cpr was given to patient for over 45 minutes. Patient was pronounced at the scene at 3:50am. Call placed to Pt family by supervisor on shift. MD to be notified. AT 3:00am, I was notified by the nurse that resident is unresponsive. Upon entering room, resident was sitting on the commode unresponsive with absent respiration and pulse. Resident lowered down on the floor with 4 person assist. CPR initiated, AED pads placed on chest with no shock indicated. 911 called and EMT and paramedics arrived around 3:10am. ACLS performed until code stopped and pronounced death at 3:48am. I called and notified family member of his demise and awaiting for family to call us back for funeral arrangements. | |
| COVID19 VACCINE (COVID19) | One week after the shot (1-14-2021) Patient (19 y.o.)reported side pain and appeared constipated, Laxatives given along with Tylenol, on further assessment Patient was noted to have left leg redness and abdominal fullness. Dr. was updated and we had orders for close monitoring, the next day when she got up, her leg appeared better, and she had passed a small BM, but by lunch she had developed significant pain and edema in her left leg, and the color of her leg was reddened again. She was sent to the emergency room with her symptoms. She was admitted back to our facility yesterday, her diagnoses included Acute provoked left external illiac, femoral, popliteal, and peroneal DVT. Elevated Factor II levels, Elevated APC resistant, May-Thurner Syndrome, history of developmental disabilities, fecal impaction and urinary retention – suspected related to her fecal impaction. Vascular surgery was consulted, and pt. was started on a heparin drip, and mechanical thrombectomy was needed for both legs due to multiple clots. She was started on Eliquis and Plavix, and thigh high compression stockings were ordered, ace wraps being used until these are supplied. Her Fecal impaction was addressed also and the urinary retention resolved. | |
| COVID19 VACCINE (COVID19) | Death | |
| COVID19 VACCINE (COVID19) | Appendicitis; Vomiting; Nausea; Chills; This is a spontaneous report from a contactable consumer. A consumer reported that her 65-year-old mother received first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 mRNA VACCINE; Lot number Unknown), into the arm at single dose on 31Dec2020 for COVID-19 immunization. The patient did not have any medical history and did not receive any concomitant products. On 31Dec2020 the same evening of vaccination, the patient experienced vomiting, nausea and chills that were considered flu like. On 03Jan2021 or 04Jan2021 her appendix ruptured. She entered emergency room on 07Jan2021 and was diagnosed with appendicitis. She was admitted to hospital on 07Jan2021. She has been given hospital care for past four days. She will have surgery in 6 weeks to repair and right now they are cleaning ruptured area. Her care team is wondering if she should get second dosage. The patient is currently hospitalized. The patient recovered from the event vomiting on 31Dec2020, recovered from the events Nausea and Chills on 07Jan2021 and was Recovering from the appendicitis. Information on the lot/batch number has been requested. | |
| COVID19 VACCINE (COVID19) | Doesn’t feel like eating; Fever; Chills/ Chilled; Nausea; Severe Headache/Dull headache/Frontal headache; Fatigue; Body aches; This is a spontaneous report from a contactable Nurse (patient). This 61-year-old female patient received the first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE, Lot number EJ1686), via intramuscular, on 06Jan2021 (at 14:30) at single dose at left deltoid for COVID-19 immunisation, administered at hospital. Age at vaccination is 61-year-old. Historical vaccine included Diphtheria and Tetanus vaccine (intramuscular, at single dose) on 15Dec2020 for immunization; and Shingles vaccine (intramuscular, at single dose) on 15Dec2020 for varicella immunization. Relevant medical history included usual tenderness. No relevant concomitant medications were provided. On 07Jan2021, she woke up at 2:00 in the morning, she had a high temperature, she was chilled, she had a severe headache, nausea, fatigue, and body aches. She got up and took ibuprofen (ADVIL). She was basically in bed, she had to cancel all her appointments in the morning, she just laid in bed and the following afternoon her fever broke at about 4:30 in the afternoon then she just had a low grade temperature and a dull headache, nausea through the next day, Friday the 08Jan2021. She still has a very dull headache and just not right, kind of like a flu bug. She had no fever; she had not had any fever after Friday afternoon or Saturday. Fever started at 2 in the morning 07Jan2021 and she experienced the chills until after fever broke. Fever went above 102 degrees. She still had a little of the nausea, she just didn’t feel like eating. She still had the dull headache. The nausea and headache have improved when compared to how it was on the 07Jan2021. She was back to work now she just has a dull frontal headache. The reporting nurse assessed all the events, except of ‘Doesn’t feel like eating’, serious for disability. She stated she may have had usual tenderness but nothing like this. The patient had recovered from the event fever on 08Jan2021 and from the event ‘chills/chilled’ on 07Jan2021; the patient was recovering from ‘nausea’ and ‘severe Headache/Dull headache/Frontal headache’, while the outcome of the remaining events was unknown.; Sender’s Comments: A possible contribution role of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE) to the onset of the reported events cannot be excluded due to temporal relationship. It is worth noting that patient had other vaccines not far ago, including Diphtheria and Tetanus vaccine and Shingles vaccine on 15Dec2020 for immunization. The case will be reassessed should additional information become available. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | his platelet levels dropped and he had a hemorrhagic stroke; his platelet levels dropped and he had a hemorrhagic stroke; This is a spontaneous report from a contactable consumer. A male patient of unspecified age received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE) via an unspecified route of administration on an unspecified date at single dose for COVID-19 immunization. The relevant medical history and concomitant medications were not reported. The patient died 2 weeks after his COVID shot because his platelet levels dropped and he had a hemorrhagic stroke. No further information provided. The autopsy was unknown. The outcome of the events was fatal. Information on the lot/ batch number has been requested.; Reported Cause(s) of Death: his platelet levels dropped and he had a hemorrhagic stroke; his platelet levels dropped and he had a hemorrhagic stroke | |
| COVID19 VACCINE (COVID19) | expired before receiving the second dose; This is a spontaneous report from a contactable nurse. This nurse reported similar death events for 8 patients. This report is for 5th of 8 patients. A patient of unspecified age and gender received the first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE) via an unspecified route of administration on 22Dec2020 at single dose for COVID-19 immunisation. The patient medical history and concomitant medications were not reported. The patient expired before receiving the second dose on an unspecified date. The patient died on an unspecified date. It was unknown if an autopsy was performed. Information on the lot/batch number has been requested.; Sender’s Comments: The limited information provided in this report does not allow a full assessment of the case. The event death with unknown cause is assessed as related to the suspect drug per company guidance. This case will be reassessed when additional information, particularly the clinical course before death, complete medical history and concomitant medication and autopsy report, becomes available.,Linked Report(s) : US-PFIZER INC-2021034595 same drug, reporter and event but different patient; Reported Cause(s) of Death: expired before receiving the second dose | |
| COVID19 VACCINE (COVID19) | passed unexpectedly; This is a spontaneous report from a contactable nurse communicated to a Pfizer colleague. This nurse reported similar death events for 8 patients. This report is for 8th of 8 patients. A patient of unspecified age and gender received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE) via an unspecified route of administration on 22Dec2020 at single dose for COVID-19 immunisation. The patient medical history and concomitant medications were not reported. The patient passed unexpectedly on an unspecified date. The patient died on an unspecified date. It was unknown if an autopsy was performed. Information on the lot/batch number has been requested.; Sender’s Comments: The limited information provided in this report does not allow a full assessment of the case. The event death with unknown cause is assessed as related to the suspect drug per company guidance. This case will be reassessed when additional information, particularly the clinical course before death, complete medical history and concomitant medication and autopsy report, becomes available. The case will be reassessed if additional information becomes available. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate.,Linked Report(s) : US-PFIZER INC-2021034595 same drug, reporter and event but different patient; Reported Cause(s) of Death: passed unexpectedly | |
| COVID19 VACCINE (COVID19) | Approximately 3 days after my injection I began experience severe tremors Ib bilateral arms, bilateral legs, head, and vocal cord tremors as well as blurry vision and memory impairment. Unfortunately, the symptoms don’t seem to be improving. My MD prescribed metoprolol, which I will begin today. | |
| COVID19 VACCINE (COVID19) | a couple hours after the vaccine, I experienced a bit of rapid heart rate, which resolved after a few minutes. The following day around 3 pm I began to have chills and felt like I had the raid heart rate again. By 5 pm I was beginning to feel really bad, I was freezing, chills and my heart rate was now extremely fast, I was having trouble speaking complete sentences, my husband drove me to the emergency department. I had a very high heart rate and high fever, I was admitted and in the hospital until Sunday afternoon. The diagnosis was pneumonia, I don’t really believe this, as I felt fine and had no symptoms prior to the onset of the fever. | |
| COVID19 VACCINE (COVID19) | Patient died 1 week after vaccination. According to family was having very rapid decline in status in recent weeks and they did not think related to vaccination. | |
| COVID19 VACCINE (COVID19) | Anaphylaxis less than two hours after vaccination. I had no symptoms immediately after vaccine however did develop symptoms within one minute of completing a run. Developed b/l hand swelling and tingling, diffuse hives and itching, tachycardia, elevated blood pressure, lips tingling and swelling which required emergency room visit and EpiPen, IV fluids, Benadryl and IV steroids. This is similar to previous reactions I have had to running previously. Symptoms resolved within one hour after treatment in ED. | |
| COVID19 VACCINE (COVID19) | Severe headaches, vomiting, dehydration, shortness of breath … led to trip to Emergency Room at Hospital on 1/16/21 at 10:45 am; diagnosis for treatment was Diabetic Ketoacidosis (DKA); patient was admitted to ICU to address critical fluid and electrolyte imbalances , headaches, body aches, dehydration, nausea, shortness of breath. DKA is medical emergency. | |
| COVID19 VACCINE (COVID19) | Pt brought to the ER with SVT. He was given 2 doses of adenosine by EMT’s. Pt was found to have hypomagnesemia and hypophosphatemia in the ER. He was admitted for observation and evaluated by Cardiology. He remained stable with NSR during admission. Pt has a follow up appt with Cardiology EP clinic on 2/18/21. | |
| COVID19 VACCINE (COVID19) | Fatigue, wheezing, soreness, palpitations | |
| COVID19 VACCINE (COVID19) | 38 year old female – healthy with no significant past medical history. Morning of 1/15/21, pt woke up with difficulty speaking (would be talking and then unable to articulate words which were replaced by grunting sounds) and tingling to her face. No changes to breathing, no numbness/tingling to extremities, equal facial symmetry. Slow onset of symptoms. Pt went to the ED, where she received a CT, MRI (inconclusive reading), lab work reported as normal per pt, EKG and chest x-ray. Symptoms self resolved while in the ED, however MD staff wanted to admit patient for 24 hours of observation and to complete an echocardiogram. Pt left AMA the evening of 1/15/21 due to resolution of symptoms and wanting to follow up with her cardiologist for the echocardiogram. Pt told by MD staff symptoms were likely caused by either TIA, possible reaction to vaccine or migraine presentation (no report of headaches/auras). Plan was to have patient on blood thinners x 30 days then baby Aspirin thereafter. Pt still needing to follow up with PCP and cardiologist for further work up. | |
| COVID19 VACCINE (COVID19) | Received vaccine on 1/16/21, on 1/17/21 started with coughing, white phlegm, SOB and on 1/18/21 developed fever to 101 and increased need for oxygen. Home requirement increased from 3L O2 to 6L O2. On 1/18/21 presented to hospital. Quickly defervesced with steroids and cefepime. Possible post-obstructive pneumonia vs immune response to vaccination. | |
| COVID19 VACCINE (COVID19) | Patient could not open and close hand after the first day of vaccination. On the third day his arm turned purple and could not be moved and was numb. A week and a half after vaccine, arm hurt and was numb. Patient was hospitalized. | |
| COVID19 VACCINE (COVID19) | Systemic: Pt monitored by nursing for 30min after inj,pt was stable/no reaction.At ~1hr post inj pt was unresponsive.Pt was a hospice/dnr per director | |
| COVID19 VACCINE (COVID19) | 9 to 36 hours. Lymphnode swelling , pain left axilla. Fever, chills ,muscle aches, brain fog. 1 week post Facial paralysis, fatigue, vocal cord weakness, feeling of unwell. | |
| COVID19 VACCINE (COVID19) | 12/28/2020: generalized weakness and fell twice at home, cough, nausea,1/04/2021: cough, nausea, fever and chronic pain when she fell from being weak. admitted to hospital with Covid pneumonia, shortness of breath, covid postive, 1/09/2021: pt on bipap, 1/15/2021: pt was intubated, on TPN, pt DNR, 1/18/2021: was extubated and put on comfort measures and passed away | |
| COVID19 VACCINE (COVID19) | Patient was vaccinated for SARS-CoV-2 on 6-Jan-21 at his site of employment, a Nursing Home. Patient presented to Urgent Care on 15-Jan-21 complaining of left sided chest pain that started the evening before with an associated slight cough. Pt was afebrile with a heart rate of 88 and an O2 sat on room air of 98% in triage. His EKG showed a sinus tachycardia of 114 with a slightly prolonged QTc of 463 ms. Physical exam was significant for bibasilar crackles and X-ray showed bibasilar infiltrates consistent with COVID pneumonia but bacterial pneumonia could not be excluded. The patients BP was documented as 97/64. He was treated with Zofran for nausea and tylenol. He was prescribed a five day course of Azithromycin, an Albuterol inhaler, guaifenessin with codeine cough syrup, and Zofran. Labs were drawn and he was discharged. His lab results were reported after his departure and were significant for a white blood cell count of 1.33, platelet count of 73, 2% myelocytes, 1% metamyelocytes, an absolute neutrophil count of 0.75 K/ul, a creatinine of 1.83, total bilirubin of 1.3, with direct bilirubin of 0.8, alkaline phosphatase of 294 and AST of 112 with ALT noted to be within normal limit. His COVID nasopharyngeal swab from the visit was reported as negative and a swab performed at his employment on 13-Jan-21 was also reported to be negative. Patient could not be reached by phone after discharge from Urgent Care about these labs. On the evening of 16-Jan-21, Police Department received a 911 call about an adult at the patient’s address who was found unresponsive. Upon arrival on scene, the patient was found to be deceased and a decision was made not to attempt to resuscitate. The death was deemed to be non-suspicious and the patient’s body was transported to a funeral home. On 19-Jan-21, I contacted the State Medical Examiner’s Office. They have decided to perform an autopsy and have recovered the CBC and chemistry specimens obtained for further testing. | |
| COVID19 VACCINE (COVID19) | 27-year-old female with past medical history of anxiety, allergic to shellfish, presented for COVID-19 vaccination, developed shortness of breath after COVID-19 Moderna injection, felt lightheadedness and noted with cyanosis as per nursing, received epinephrine injection and transferred to ED. In ED she received solumedrol, benadryl and pepcid. Vitals in the ER Revealed tachycardia HR 95-105 , Sat 96% on room air not in distress. Patient was admitted for further observation | |
| COVID19 VACCINE (COVID19) | 1. Shaking 2. Whole body tingling 3. Left arm tense (injection was provided in right arm) 4. Felt clammy Walked over to hospital attached to the facility and was discharged the same day. All symptoms resolved. | |
| COVID19 VACCINE (COVID19) | “Patient called this nurse stating she had an allergic reaction to COVID vaccination given on Friday 1/15/21. States she felt fine for the 15 minutes post immunization, was on her way home and started feeling dizzy, short of breath, chest heavy, throat felt full “”like a ball in it””. She came back to clinic which was closed but sat in the parking lot for a while. While in parking lot trying to figure out what to do, her symptoms lessened. She got home safely but started to feel jittery/shaky and her BP was very high (couldnt remember exact number). She then went to urgent care where they told her she was having an allergic reaction and given a pill of something and steroid for 6 days. Went home from urgent care and BP still high but got better at bedtime. Saturday she had a “”really bad headache and just layed around all day. I was not able to function at all.”” Sunday she still had a headache and added muscle aches. Monday she started feeling “”a lot better”” until 8 PM when she was walking around doing her nightly routine and started to feel a wave of dizziness, throat felt funny so she sat down and took her BP with result of 207/131. Says this reaction felt worse than Friday’s reaction so she went to ER where she was again told she was having an allergic reaction and the steroid given to her at Urgent Care was not helping and to stop taking them. Given Benadryl in the waiting room, had labs and EKG which came back “”normal””, and given a different med Vistaril to take with any future symptoms. Was also told to NOT take the second dose of COVID vaccination. Says she has not had to take the Vistaril yet and has not had any sign of reaction today so far. Said she did report the initial headache on the V-safe app.” | |
| COVID19 VACCINE (COVID19) | Anaphylactic |
| COVID19 VACCINE (COVID19) | At approximately 4pm on Jan 11, 2021, I began to have hard chills and fever that reached 104.9. I was admitted to ICU at the Hospital. My blood pressure dropped to dangerous levels. I was diagnosed with sepsis and the doctors determined it was caused by the vaccine. | |
|---|---|---|
| COVID19 VACCINE (COVID19) | Pulmonary Edema, fever, nausea, vomiting | |
| COVID19 VACCINE (COVID19) | Moderna COVID- 19 Vaccine. Vaccine recipient reported on 1/19/2021 that they received the Moderna Vaccine on 1/8/2021. The following week on 1/15/2021, they reported while driving, their area around their right eye became numb and they began to have blurry vision. The numbness spread to around their face/mouth. They pulled over and their spouse drove him to the hospital. Roughly 20 minutes after the initial symptoms, they developed chest pain and patient reported that the ED noted an abnormality on their EKG. The patient had to be admitted overnight for observation. The patient reported that on 1/18/2021, they still had mild chest pain and facial numbness remains around the right eye, left mouth/cheek area, and tongue. They did develop fever and a headache. The patient reported that on 1/19/2021, they are waiting on results, additional testing, and further follow-up appointment with their provider. | |
| COVID19 VACCINE (COVID19) | Visited Provider appx 500 pm 1.14.2021 DVT – left calf – 2 clots via ultrasound on Eliquis now | |
| COVID19 VACCINE (COVID19) | Lacunar infarct (CVA) of right thalamus | |
| COVID19 VACCINE (COVID19) | Patient developed symptoms of Guillain-Barre syndrome on January 15, 2021 and was admitted the Hospital. She was diagnosed and eventually required ICU level care and has been treated with plasmapheresis. She is currently still in the ICU but is stable. | |
| COVID19 VACCINE (COVID19) | Family was told that Patient expired in his sleep during the early morning hours of 1/15. I spoke with him the evening before (on 1/14), which was a day after he had received the Covid vaccine. He was not having any symptoms of allergy or reaction then. He did say that he felt tired, but he often complained of feeling tired over time. | |
| COVID19 VACCINE (COVID19) | Hypoglycemia(40mg/dL) and required ICU admission. | |
| COVID19 VACCINE (COVID19) | Resident was noted unresponsive, no respiration, no blood pressure, no pulse, code blue called according to facility protocol, resident is full code, CPR started, 911 called, arrived and took over from staff. Resident was pronounced dead at 1:16pm 1/18/21 | |
| COVID19 VACCINE (COVID19) | “””Patient states that he received the Covid vaccine today on left arm. Immediately after receiving it, felt his left arm went numb, then felt his lips on the left side going numb. Sensation progressed to his whole face, and down his neck, and back down to the whole left arm. He states that he even felt his truncal area, kidney, and part of his right foot going numb. States that he went to the ER for further evaluation, and while waiting there for about an hour, the sensation resolved. He denies any tingling or painful sensation. Does not think he was weak at the time.””” | |
| COVID19 VACCINE (COVID19) | Resident was found deceased in his bed at 7:15 am. | |
| COVID19 VACCINE (COVID19) | Tachycardia, Shortness of breath, headache, dizzyness, weakness, chills, nausea, fever | |
| COVID19 VACCINE (COVID19) | Dizziness, Headache, Myalgia, Tachypnea, CoughWheeze, NauseaVomiting, Palpitations & Tachycardia & Narrative: Patient stated that after receiving injection on 01/06/2021, tasted metal in her mouth. No reaction noted in clinic after vaccine administered. Patient states that after returning home, she began to have chills, headache, and muscle aches. Could not sleep. On 01/07/2021. Patient continued to experience above symptoms. Approx. 13:50 on 01/07/2021. Patient presented with respiratory difficult, tachypnea stridor, and stated she felt as if her airway was closing. Patient was vomiting and was tachycardic. Epi-pen administered via left lateral thigh. Patient administered 50mg of PO Benadryl, and 2 puffs of albuterol inhaler. Continuous V/S initiated. Patient began to experience relief of symptoms. HR and blood pressure remained elevated, but this was expected side effect of epi. SpO2 stabilized around 99% on room air. Patient was monitored for 60 minutes. Transportation home was arranged and family was present to observe overnight. | |
| COVID19 VACCINE (COVID19) | mi Narrative: patient with asymptomatic covid 19, covid positive 12/10/2020. | |
| COVID19 VACCINE (COVID19) | dose given 1/13/21, patient hospitalized with high blood sugar, hyperkalemia, hypernatremia on 1/15/21 after being lethargic with shallow breathing. Patient still hospitalized as of 1/19/21 and diagnosed with Diabetes | |
| COVID19 VACCINE (COVID19) | Severe dissociative event (psychotic break) beginning <72hr after administration of vaccine, and continuing another 48 hours before resolution. Patient has no prior adverse psychiatric history. Transported to local ER on Monday 11th upon worsening of condition. Administered Haldol in the ER as a sedative after becoming combative during dissociative state. Patient woke up Tuesday the 12th with recurrent, but significantly diminished, dissociation, which had largely resolved by late Tuesday. Patient transported to Mental Health on a 5150. Released Friday the 15th around noon. No recurrent symptoms since. | |
| COVID19 VACCINE (COVID19) | Resident reported she didn’t feel well. She started running a fever of 103. Resident complained her stomach and genitals were burning and pain in her legs. Resident has been vomiting today and diarrhea. EMS was called and resident transported to Hospital today 1/19/21 | |
| COVID19 VACCINE (COVID19) | COVID 19 vaccine, unknown which company Chronically ill in a skilled nursing facility found diaphoretic, hypotensive, hypoxia to 85% arrived to Emergency dept in cardiac arrest Died within 65 minutes of nursing finding patient in distress Wife felt it may have been related to vaccine date of vaccination 1/6/20 hx covid 19 PNA in April 2020 | |
| COVID19 VACCINE (COVID19) | hypoxia, secretions,cough, dyspnea Narrative: ALS patient on hospice with ongoing history of aspiration pna, receiving tube feeds. Developed incr in secretions, hypoxeia, temp and with recently noted clogged feeding tube. | |
| COVID19 VACCINE (COVID19) | pneumonia Narrative: On 11/9/20, Patient had a presumptive positive COVID (COBAS) screen as part of routine CLC screening and then on 11/13/20, he had a repeat COVID (CEPHID) that was negative. Then on 12/22/20, he received his first COVID vaccine. On 12/26/20, he began to have c/o hurting all over. Noted history of aspiration and COPD. On 12/29/20, he began to have coughing, increased shortness of breath and runny nose with course breath sounds in his bilateral lower lobes. A chest xray was done and he was initiated on oral azithromycin and cefepime for a bilateral pneumonia. On 1/3/21, he continued to decline with increasing shortness of breath and was subsequently transferred to acute care medicine. All COVID tests have been negative since the presumptive positive on 11/9/20. He did have a CTA that ruled out PE but did show bilateral pneumonia. His antibiotics have been changed to meropenem, vancomycin and IV azithromycin. He remains on acute care at time and has not required ICU care. | |
| COVID19 VACCINE (COVID19) | COVID positive Narrative: Patient is a resident and received his first COVID vaccine on 12/28/20. On 1/4/21, he had a COVID routine screen done that returned positive on 1/6/21. Per notes, he was asymptomatic at the time; however for isolation purposes, he was transferred to acute care medicine services. On 1/7/21, he was noted to have a slight increase in BUN/creatinine ratio thought to be due to volume depletion and has been ordered IV fluids. He still remains free of any respiratory symptoms at this time. | |
| COVID19 VACCINE (COVID19) | The patient had severe shortness of breath resulting in cardiac arrest on the 5th day after the vaccine. Shortness of breath started 12 hours after injection. On the 5th day, the patient was discovered to also have a rash throughout his body, but it is unknown when this rash started. | |
| COVID19 VACCINE (COVID19) | Diarrhea & NauseaVomiting Narrative: | |
| COVID19 VACCINE (COVID19) | Pt is 39 y/o female. Pt is casual RN for ED. Pt received COVID-19 vaccination here on the 23rd of December. Pt began feeling weak on the 17th of January. On the 18, Pt began experiencing numbness and tingling in hands and feet. Pt has been seen at facility and her PCP prior to coming to ED. Pt PCP called me and told me she is concerned that the Pt might have Guillain Barre syndrome and referred her here. Pt now c/o numbness and tingling from feet to mid abdomen and numbness/tingling up entire arm. IV was established and labs drawn, CT Head normal. Pt to IR for LP for definitive Dx of Guillain Berre. Pt admitted to room 202. Director of ED notified. | |
| COVID19 VACCINE (COVID19) | COVID-19 Vaccine | |
| COVID19 VACCINE (COVID19) | Sudden death without warning symptoms 4 days after vaccine. Many medical problems which most likely explain the outcome but spouse feels it is related and it is a new vaccine. Monitor for pattern? | |
| COVID19 VACCINE (COVID19) | Excruciating abdominal pain, left arm pain, chest pain. Gangrenous appendicitis requiring emergency surgery and followed by admission for complicated acute abdomen. | |
| COVID19 VACCINE (COVID19) | “Resident experienced chest pain the evening he received the vaccine and requested to go to the hospital as he stated his “”chest is pounding””.” | |
| COVID19 VACCINE (COVID19) | Resident received 1st on 1/11/21 at 12:10am (1/12/21) resident was found unresponsive. Code Blue, 911 called at 12:11am. FD and EMS arrived, resident pronounced at 12:51am. | |
| COVID19 VACCINE (COVID19) | 24 hours after the vaccine administration, patient began experiencing respiratory (asthma) symptoms. She was treated with nubulizer treatment and albuterol HFA inhaler. | |
| COVID19 VACCINE (COVID19) | Hypotension, Prolonged seizure with bowel incontinence, cough, weakness and delirium, resulting in 911 transport and admission to hospital for intubation and mechanical ventilation for acute respiratory hypoxia. | |
| COVID19 VACCINE (COVID19) | Appendicitis | |
| COVID19 VACCINE (COVID19) | Pregnant at the time of vaccination; Pregnant at the time of vaccination; Miscarriage (symptoms started 08Jan2021, confirmed 10Jan2021); This is a spontaneous report from a contactable physician (patient herself). A 37-year-old female patient received her first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE, lot number and expiry date were unknown), via an unspecified route of administration on the right arm on 06Jan2021 12:30 at a single dose for COVID-19 immunization at the hospital facility. The patient had no relevant medical history and no known allergies. Concomitant medication included azelastine;fluticasone nasal sprays, prenatal. The patient did not have any other vaccine in four weeks. She did not have COVID prior to vaccination. The patient was 6 weeks pregnant at the onset of the event. Last menstrual date was on 11Nov2020 and Gestational period was 7. The patient was due to deliver on 26Aug2021. The patient reported miscarriage (symptoms started 08Jan2021 06:00 PM, confirmed 10Jan2021). The event resulted in doctor or other healthcare professional office/clinic visit, Congenital anomaly or birth defect. Treatment and event outcome were unknown. The patient had not been COVID tested post vaccination. Information on the lot/batch number has been requested.; Sender’s Comments: Based on a close compatible temporal association, contributory role of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE) to patient’s miscarriage cannot be completely excluded. The case will be reevaluated should additional information become available. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | “Hypertensive Emergency (BP 219/114) with no previous blood pressure issues; Radiating chest pain, left arm pain; jaw pain; This is a spontaneous report from a contactable other Health Professional (patient). A 40-year-old non-pregnant female patient received the first dose of bnt162b2 (PFIZER-BIONTECH COVID-19 VACCINE, lot number: EX5170), via an unspecified route of administration on 16Dec2020 15:00 at single dose in left arm for covid-19 immunization. Medical history included symptomatic PVC’s (Premature ventricular contractions), tachycardia, bradycardia, CVA (cerebrovascular accident) from 2018 to an unknown date, asthma and rhythm. Concomitant medication included flecainide, spironolactone, metoprolol for rhythm. The patient had known allergies included hydrocodone bitartrate, paracetamol (VICODIN), eletriptan and adhesive. Prior vaccination, the patient had no covid. On 22Dec2020 18:00, the patient experienced hypertensive Emergency (BP 219/114) with no previous blood pressure issues. Radiating chest pain, left arm pain, and jaw pain. Admitted to the hospital where an echocardiogram and angiogram was performed showing clear coronary arteries and no hypertensive remodeling of the heart. Issue has been ongoing since, despite interventions. The events result in emergency room/department or urgent care and hospitalization from an unspecified date for 1 day. The patient received the treatment for the events included frequent nitroglycerin, hydralazine and metoprolol. The patient underwent curative-SARS-Cov-2 Assay RT-PCR on 01Jan2021 with negative result. The outcome of the events was not recovered.; Sender’s Comments: Based on the information available, contributory role of BNT162B2 ((PFIZER-BIONTECH COVID-19 VACCINE,) to event “”hypertensive emergency (BP 219/114) with no previous blood pressure issues”” cannot be excluded. The events chest pain and pain in jaw are attributed to underlying medical conditions and assessed unrelated. BNT162B2 ((PFIZER-BIONTECH COVID-19 VACCINE,). The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate.” | |
| COVID19 VACCINE (COVID19) | I am a registered nurse at hospital. On 12/25, seven days after receiving the shot I started to get right lower leg pain and I kept complaining about it till New Years Day. I had no symptoms of a DVT. I triaged on 1/1/21 and the doctors ordered labs/imaging and the results were as followed: D-Dimer biomarker (+) , Ultrasound of the Rt lower leg ( – ) , CTA showed a PE (segmental right upper lobe pulmonary artery consistent with pulmonary embolus). I was discharged on Xarelto and advised to follow up with a hematologist. On 1/5/2021, I went to hematology and they did a whole bunch of labs. I was sent to get a ultrasound of the leg because the pain persist and they found a clot hidden by my soleus. The plan is to continue on the Xarelto for 6 months. Come back in 3 weeks to scan my leg again and get my lab results. On 1/12/2021, I received the 2nd shot of the Pfizer vaccination. | |
| COVID19 VACCINE (COVID19) | “Client was administer 2nd dose of Moderna COVID-19 vaccine at 1:32pm. Client reported has “”sore arm from first dose””, but denied any other issues from 1st dose. At 1:44pm, client reported was feeling flushed and slightly dizzy/light headed. Client appeared flushed, clammy and was slightly confused. Client was sitting in chair and was assisted to the floor and clinical assist code was called. VS were: BP: 149/84 and pulse ox was 97% on RA. Initially, HR was in the low 30’s, but after lying down, came up to 86 and stayed in the 80-90’s during assist. Client began to report chest tightness and feeling foggy and remained slightly confused and at times speech was garbled. Client denies sensation of throat closing and denied shortness of breath. Client was assisted to a cot and transported by ER staff and MD to the ER for evaluation.” | |
| COVID19 VACCINE (COVID19) | Presented to Urgent Care for weakness and confusion, transferred to ED, patient had a cardiac arrest and was unable to be resuscitated | |
| COVID19 VACCINE (COVID19) | “””I received the Pfizer Covid vaccine Wed afternoon around 4pm. Thursday morning around 9:30 I started with severe pain in my left leg. The pain worsened through the day and my leg began swelling. No other symptoms at all. This morning my leg was twice the size of my right leg so I went to the ER. I live in so I’m at ED. I have a massive blood clot running the the length of my leg – from my thigh to my ankle. I’m very lucky I got here so fast! I?m a very healthy 49 year old with no history of DVT or blood clots so they dug further to find out why. A cat scan showed I have a congenital condition called May Thurner Syndrome. I?m so relieved to have an answer and it?s fixable! The vascular doctors are not 100% convinced that?s not all that was going on as I was born with the syndrome and I?ve gone this long without a clot. So they are doing lots of labs to see if anything else shows up. This is where we are at. I?m being admitted to take care of the clot.””” | |
| COVID19 VACCINE (COVID19) | Pt woke up with tongue swelling morning following her vaccine. Was admitted for angioedema. | |
| COVID19 VACCINE (COVID19) | Rapid heart Rate that began about 12 hours after the injection. Heart rate of 123 all night and went to ER next morning after calling Nurse on Call system. I was admitted and the Dr ordered bag after bag of fluids to and kept me in the unit overnight for observation. Did may hear tests (EKG, ECHO STRESS,CTA chest) and results all came back good. I was released on 1/19/21 at 2pm and my hear rate is back to normal (82 bpm). | |
| COVID19 VACCINE (COVID19) | Extreme fatigue since getting shot – effecting ability to work | |
| COVID19 VACCINE (COVID19) | Started with cough, mild shortness of breath and feeling terrible in evening of 1/19. | |
| COVID19 VACCINE (COVID19) | Death 3 days after receiving 2nd dose of COVID vaccine, unknown if related to vaccine administration. | |
| COVID19 VACCINE (COVID19) | Approximately after 20 minutes after vaccine administration, my throat felt numb and i could not swallow my saliva, no acute breathing difficulty , I could not swallow water , I was very anxious and went back to facility I got vaccine , The attending doctor administered Benadryl I 50 mg IM , and then EPI 0.3 mg . EMT was called , and ED at Hospital i was attended , and Solumedrol 1.25 mg was administered IV and Pepsid for GERD , i also had, I was observed for a few HRs and skript for Prednisone and Benadryl was issued. I was then feeling better, | |
| COVID19 VACCINE (COVID19) | My arm was a little sore after the vaccination but no other symptoms. And on 12/30 I woke up with a sever fever, vomiting and diarreah. Went to the ER and was diagnosed with CHF because my feet were so swollen and was given lasix and released. I continued to feel bad so on that Sunday 1/3 I went back to the hospital and was admitted and tested positive for COVID-19. I spent from Sun-Wed in the hospital | |
| COVID19 VACCINE (COVID19) | Patient has end stage renal disease and rapidly worsening dementia, family could no longer care for him at home, and he was admitted for 14-day quarantine prior to admission to inpatient hospice. Received vaccine on 1/12 without apparent adverse reactions. Patient started refusing oral intake on 1/16, and CMP on 1/17 showed hypernatremia 165 (new issue). His BUN 138 CREAT 6.93 K 5.2 were his baseline. He was found to be deceased on 1/18 at 11:18 pm. | |
| COVID19 VACCINE (COVID19) | Pt found unresponsive at home, respiratory distress. Had reported nausea and vointing for two days prior to admit which started 1/15. Acute metabolic encephalopathy and acute renal failure Currently at time of this report still in critical care | |
| COVID19 VACCINE (COVID19) | Shaking and then became unresponsive | |
| COVID19 VACCINE (COVID19) | Headache and stoke | |
| COVID19 VACCINE (COVID19) | Stroke-like symptoms approximately 2-3 hours after receiving shot (aphasia), BP bottomed out, was transported by EMS and is currently on a ventilator in hospital. CT scan clear; MRI pending. | |
| COVID19 VACCINE (COVID19) | The next morning I felt chills, really cold, my arm never hurt at all. I was freezing. I had no energy. Very lethargic, with a blanket around me. Never had a fever. All of a sudden, around 2:30PM all dissipated, it was all gone and I was fine. A week later I called my PCP because the symptoms came back – the lethargy. He suggested me to go to the ER. I went and could barely write my name on the sign in sheet at the hospital. They did 5 COVID tests and 4 of them were negative. I was at the ER for 3 days and finally was admitted and stayed for 11 days. I was sent home with oxygen and my levels are finally getting back to 92/93. I can’t walk at this time. I lost 17 lbs and if I try to walk my lungs shut down. ( I went to the ER n 01/07 and was discharged on the 17th – I was also at the ER the week before when they tested me and it kept coming back negative) My pulmonary MD | |
| COVID19 VACCINE (COVID19) | death by suicide Narrative: death by suicide; 12/26/20, self inflicted gun shot wound; found deceased by family member | |
| COVID19 VACCINE (COVID19) | Started with HA/fever/fatigue and body aches on date of vaccine on 1/5/21. Also had shakes,and had numbness loss of feeling to leg leg. Was admitted to Hospital for 5days. Had intermittnet seizure type episodes. Has had labs and imaging tests that have been neg Under the care of the A Neuro Group. MRI scheduled for 1/20/21. As of 1/20/21 feeling better but still weak. | |
| COVID19 VACCINE (COVID19) | Fainting, dizziness and weakness, trembling, BP 168/129. HR 145 | |
| COVID19 VACCINE (COVID19) | Patient received Vaccine and during observation period began starring forward and not responding to staff. Patient was taken to the emergency room and evaluated and noted to have a flat affect and general weakness. Patient did not loos consciousness or have any signs of distress. Was admitted to the hospital and noted that she had been in out of country for a week (possibly had cosmetic surgery) | |
| COVID19 VACCINE (COVID19) | Around 10pm on Tuesday I started to have severe neck pain, headache, fatigue. I ended up going to the emergency room after my shift but due to the physicians recommendations I deferred the lumbar puncture while there. Later Thursday night, the neck pain continued, and I started having fevers with a max of 102.1 so I returned to the ER the following day for the lumbar puncture. I was admitted to the hospital for aseptic meningitis. | |
| COVID19 VACCINE (COVID19) | severe temporary paralysis from the neck down. | |
| COVID19 VACCINE (COVID19) | Resident was noted to have increase weakness on 1/15/2021. Resident was warm to touch with low grade fever of 99.3 F. Resident was up propelling self in w/c on 1/16/2021 he was pleasant, accepted medications and ate lunch. He was found slumped over in his w/c not responding and vital signs absent. | |
| COVID19 VACCINE (COVID19) | Pt was 18 weeks pregnant at the time of the vaccine. Second pregnancy. Pt is a physician. Pregnancy was entirely normal up to that time. On 1/18/2021, she began to have heavy vaginal bleeding probably due to a placental abruption and subsequently delivered at 18 weeks. Baby was stillborn. Ultrasound done 1/15/2021 normal. Lethal event for the fetus. The patient did well. | |
| COVID19 VACCINE (COVID19) | Hypotension/ hypotensive; Hypoxia/ hypoxic; Tachypnoea/ tachypnoeic; Unresponsive to stimuli/ unresponsive; Death; Somnolence/ Drowsy; Hypoglycaemia/ hypoglycaemic; Hypothermia/ hypothermic; Hypophagia/ reduced oral intake; Fall; Confusional state/ confused; Headache; Chills; Skin ulcer/ Leg ulcers; Oedema peripheral/ bilateral leg oedema; Oxygen saturation decreased/ low saturations; feel unwell; This is a spontaneous report from a contactable physician downloaded from the Regulatory Agency, manufacturer report number GB-MHRA-ADR 24566650. A 95-year-old female patient received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE) via an unspecified route of administration on 16Dec2020 at single dose for COVID-19 immunization. Medical history included atrial fibrillation from an unknown date and unknown if ongoing, moderate aortic stenosis from an unknown date and unknown if ongoing, pulmonary hypertension from an unknown date and unknown if ongoing, possible papillary fibroelastoma from Jun2019 and unknown if ongoing, non-specifically lethargic from Dec2020 and unknown if ongoing. The patient had been non-specifically lethargic for 1-2 weeks in early Dec2020. The patient’s concomitant medications were not reported. In the 24-48 hours following vaccine, developed headache and chills. Daughter thought it was all post-vaccination inflammatory response and that it would settle. Continued to feel unwell, reduced oral intake, 2x falls and became confused on 25Dec2020. The patient was admitted on 26Dec2020, hypothermic. Consultant review 27Dec2020 and no diagnosis documented. Leg ulcers and bilateral leg oedema noted, supplemental oxygen commenced due to low saturations in Dec2020. Plan was for chest x-ray, infection screen, COVID test. Drowsy and found to be hypoglycaemic overnight 28Dec2020 to 29Dec2020, given intravenous treatment and blood sugars improved. National Early Warning Score (NEWS) of 14 (hypotensive, hypoxic, tachypnoeic, unresponsive) on 29Dec2020 and Medical Emergency Team (MET) call put out at 06:50. By the time MET team arrived the patient had died. The patient experienced headache on Dec2020 , chills on Dec2020, hypophagia on 25Dec2020, fall on 25Dec2020, confusional state on 25Dec2020 , hypothermia on 26Dec2020, skin ulcer on Dec2020, oedema peripheral on Dec2020, oxygen saturation decreased on Dec2020, somnolence on 28Dec2020, hypoglycaemia on 28Dec2020, hypotension on 29Dec2020, hypoxia on 29Dec2020, tachypnoea on 29Dec2020, unresponsive to stimuli on 29Dec2020, death on 29Dec2020 , feel unwell on 25Dec2020. All the events except feel unwell were reported as serious as hospitalization and death. The patient underwent lab tests and procedures which included computerised tomogram head: no bleed, C-reactive protein: 37, echocardiogram: possible papillary fibroelastoma- not investigated in Jun2019, white blood cell count: normal. The patient died on 29Dec2020. An autopsy was not performed. The outcome of the event feel unwell was unknown, while other remain events was fatal. No follow-up attempts are possible; information about lot/batch number cannot be obtained.; Reported Cause(s) of Death: Headache; Chills; Fall; Hypophagia/ reduced oral intake; Confusional state/ confused; Skin ulcer/ Leg ulcers; Oedema peripheral/ bilateral leg oedema; Oxygen saturation decreased/ low saturations; Somnolence/ Drowsy; Death; Hypoglycaemia/ hypoglycaem | |
| COVID19 VACCINE (COVID19) | sinus arrhythmia; Night sweats; Heart rate low; Dyskinesia; This is a spontaneous report from a contactable nurse (patient). This 36-year-old female patient the first dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE), intramuscular at single dose in the left arm on 07Jan2021 10:00 for covid-19 immunisation. Medical history included hypotension, rheumatoid arthritis, asthma. Concomitant medication included clonazepam, methylprednisolone. On 07Jan2021 21:00, the patient experienced sinus arrhythmia, night sweats, heart rate low, dyskinesia, all with outcome of recovered with sequelae. Therapeutic measures were taken as a result of the events included Inderal 10mg. The events were assessed as congenital anomaly. The patient did not receive any other vaccines within 4 weeks prior to the COVID vaccine. The patient was not diagnosed with COVID-19 prior to vaccination. The patient had not been tested for COVID-19 since the vaccination. The patient was not pregnant at the time of vaccination. Information on the lot/batch number has been requested.; Sender’s Comments: A possible contributory effect of suspect BNT162B2 on reported events cannot be excluded. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to regulatory authorities, Ethics Committees, and Investigators, as appropriate. | |
| COVID19 VACCINE (COVID19) | Several back operations of stimulator in the back and in pain management; This is a spontaneous report from a contactable Consumer. This adult female Consumer(patient) reported that: An adult female patient received bnt162b2 (BNT162B2) at single dose on an unspecified date for Covid-19 immunisation. Medical history was none. No known allergies. The patient’s concomitant medications were not reported. Patient was not pregnant a time of vaccination. The patient had not received any other vaccines within 4 weeks prior to the BNT162B2 vaccine. The patient had not experienced Covid-19 prior to vaccination. The patient experienced several back operations of stimulator in the back and in pain management on an unspecified date, resulted in disability or permanent damage. Post the vaccination, the patient has not been tested for COVID-19. The outcome of events was unknown. Information on the lot/batch number has been requested. | |
| COVID19 VACCINE (COVID19) | “suddenly lost mobility of left arm; Continue paresthesia and proprioreceptive deficits of left arm; Continue paresthesia and proprioreceptive deficits of left arm; This is a spontaneous report from a contactable nurse (patient). This 46-year-old female patient received the first single dose of BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE; lot EK5730) intramuscular, in right arm, on 16Dec2020 at 14:00, for COVID-19 immunization. No other vaccine was given in 4 weeks. Medical history included hypothyroidism, migraine headaches, IBS and COVID-19 (on an unspecified date prior to vaccination). Past drug history included allergy to morphine. Concomitant medication included levothyroxine sodium (SYNTHROID). On 23Dec2020 at 09:30 the patient experienced suddenly lost mobility of left arm, continue paresthesia and proprioreceptive deficits of left arm. She was transported to the ER and was admitted to hospital for 2 days. CT of brain X3, MRI of brain X2, MRI of C-spine and Brachioplexus were performed with unknown results. The patient was not tested for COVID19 after vaccination. The events resulted in: Doctor or other healthcare professional office/clinic visit, emergency room/department or urgent care, hospitalization, disability or permanent damage. No treatment was administered. The events had not yet resolved.; Sender’s Comments: Based on the temporal relationship, the association between the events “” lost mobility of left arm, continue paresthesia and proprioceptive deficits of left arm”” with BNT162b2 can not be fully excluded. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to regulatory authorities, Ethics Committees, and Investigators, as appropriate.” | |
| COVID19 VACCINE (COVID19) | on 1/12 started body aches and chills, took Tylenol and felt better, Wednesday felt short beat and irregular and heart beat not regular. checked pulse and it was irregular, that night 9-9:30 I went to ER close to house and pulse and heart rate abnormal after 2 hours went home and advised to contact cardiologist, he said to come in Thursday and the irregular beats were off and on atrial flutter, on Friday I came home and went back to hospital and got the shot cardio version or ablation to revert the rhythm and because the rhythm was back and forth I was observed until released on 1/19/21. Surgery was in 2017 and never had problems and they stated heart was compromised and maybe this is your reaction to the vaccine. | |
| COVID19 VACCINE (COVID19) | The patient was seen in my office on 1/19/21 with complaint of heavy vaginal bleeding. A CBC was obtained which revealed an H/H of 12.2/36.1 and a platelet count of 1 (not 1K, but 1 platelet!) and this was confirmed on smear review. She was immediately sent to the Hospital ED and repeat CBC confirmed the critically low platelet count. She is currently hospitalized and she has received platelet transfusions but her platelet count is still critically low. She is also receiving steroids and immunoglobulin and is under the care of MD (Heme/Onc) | |
| COVID19 VACCINE (COVID19) | Resident became lethargic, general weakness outside baseline, unable to walk, bumbled speech. Elevated HR and Temp of 105.2F | |
| COVID19 VACCINE (COVID19) | Death on 1/15/2020 | |
| COVID19 VACCINE (COVID19) | Patient developed right facial numbness and facial droop on 1/7/21. She came to the TMH ED and was admitted. She was afebrile; neurologic exam was consistent with a peripheral facial palsy on the right. | |
| COVID19 VACCINE (COVID19) | Sudden Death within 24 hours of vaccine |
| COVID19 VACCINE (COVID19) | Hemorrhagic Stroke, Right Basal Ganglion | |
|---|---|---|
| COVID19 VACCINE (COVID19) | 12:00 noon my arm was super sore and swollen and I could barely raise it. By the evening on the 24th I was super tired. Christmas , the 25th,I was really fatigued. 26th, I was very fatigued and no energy – like I almost had the flu. 27th – the same – couldn’t get out of bed. 28th – started getting a dry, hacky cough and then it went away. 29th – tried to go to work and I didn’t feel good at all and I just wasn’t feeling myself. Had brain fog. Went to work – things that I know that Know wasn’t there because of brain fog. Felt really like flu symptoms that night. 30th – body aches; headache and eyes felt like pressure behind them. Sent me to COVID and it was positive – was out 2 weeks from work. Went to back to work last week – but I still had shortness of breath, brain fog and fatigue and cough on Moday the11th; super tired still and I kept pushing myself to try to work and by Friday, 15th, I was just done. Exhausted. 16th – still having fatigue and 17th I thought I felt better when I woke up but still had my shortness of breath, cough and brain fog. At 2:00 pm I started feeling dizzy and faint like. I ate and within 20-30 min after food started throwing up, pounding headache. Every time I started trying to drink I would throw up. Monday, 18th, I went to work and I noticed whenever I moved around I was dizzy and short of breath and I couldn’t eat or I would throw up. So slept the rest of the day. About 4:pm on Monday I thought I was going to pass out. 02 level at 86. Heartrate – Palpitations at 130. It was bouncing. Hving chest pressure, dizzy, pounding heart – 02 86 and Heartrate145. ER at 5:00 pm at Medical Ctr. Nausea medicine: Phenergan and Zofran. Did IV to hydrate me as I was dehydrated. Gave me Pepsid. I was a direct admit. They kept giving me hydration and nausea med and med for headaches and cardio workup – cardio came back normal. Severe lung inflammation. Was in the hospital until evening of 19th. | |
| COVID19 VACCINE (COVID19) | Patient woke apx 0200 complaining of nausea to group home staff. Vitals were checked at that time and WNL. Patient went back to bed. When staff went to wake patient apx 0530, he was unresponsive and had no pulse. Chest compressions were started and EMS called. | |
| COVID19 VACCINE (COVID19) | Patient got her 2nd dose of Pfizer covid vaccine on 1/8. On 1/11 she had intermittent chest pain that lasted a few days and started to notice small purpura rash on left breast. She didn’t think much of it but noticed the same type of rash on her pant line and then right thigh. On 1/15 she called Occupational Health who advised her to go straight to the ED. | |
| COVID19 VACCINE (COVID19) | On 1/9/2021 observed with elevated respirations of 38-42 per minute, BP manually 72/50. pulse is jumping rapidly between 110-16 bpm. oxygen sat 76% RA, resident refusing oxygen at first attempt, allowed oxygen to be placed, is now 84% on 4L. resident shaking head yes that he is hurting, and yes that he would take medication for pain. Dr. notified, branch block. Received order for morphine 2mg per hr as needed for elevated respirations and pain. Dr. also gave orders to D/C Tamsulosin and finasteride. Resident continue with decreased O2 sats and elevated respirations. Absence of vital signs on 1/10/21 at 826PM. | |
| COVID19 VACCINE (COVID19) | Jan 4, 2021 received shot at work in morning around 11:00 am- felt fine at work all day. Around 7:00 that night began to have back pain, fever reached up to 102 around 2:00 am and took Tylenol. Felt very weak, headache, difficulty walking and decreased balance. On Jan 5, 2021 still had back ache and headache. On Jan 6, 2021 I felt better and began to have abdominal pain after eating dinner. I assumed it may be because of eating more then I had in past few days and did not attribute to much but the pain gradually increased to a strange burning/pressure across my upper abdomen that night. Very nauseous and ran low grade fever (99.45). Next morning I had less diffuse pain and could localize it to right lower quadrant. | |
| COVID19 VACCINE (COVID19) | Tinnitus started in right ear within hour after receiving first vaccination but resolved within a couple of day. Within 24 hours of receiving second vaccination had muffled hearing, Jan 3, 2021. Symptoms were ignored thinking they would resolve. When symptoms persisted and evaluated patient was noted to have a severe right sided low frequency hearing loss with poor word recognition score. Patient was started on high dose steroids with partial recovery of symptoms. | |
| COVID19 VACCINE (COVID19) | Idiopathic intracranial HTN – IIH | |
| COVID19 VACCINE (COVID19) | Unknown as to any correlation with vaccine as this was a hospice patient that was already experiencing decline. Patient became Jaundice for approximately one week prior to expiring. | |
| COVID19 VACCINE (COVID19) | Unrelenting headache, chills, nausea, body aches, fever of 101.3F. Onset 14 hours after vaccine. Fever 20 hours after. Relieved with Tylenol and Motrin, ice packs. | |
| COVID19 VACCINE (COVID19) | Patient received COVID 19 vaccine 01/14/2021. Patient died in his sleep 01/16/2021. | |
| COVID19 VACCINE (COVID19) | Patient received COVID-19 vaccination on 1/14/2021. On 1/17/2021, patient was transferred to Hospital s/p multiple cardiac arrests. Patient was hyperkalemic and in acute renal failure at time of transfer. Hyperkalemia was treated, but the patient suffered PEA vs VFib. At the time of transfer, patient was on vasopressin, norepinephrine, and epinephrine. The patient had an EF of 40-45% and elevated troponins. Patient was made DNR and placed on comfort care. Patient passed away on 1/18/2021. Ultimately we suspect that the patients condition was a direct result of his underlying disease states, but wanted to make sure reporting was made available. | |
| COVID19 VACCINE (COVID19) | Thursday 1/6/21 body aches, fever, chills Fri, Sat, Sun- Vomiting and diarrhea. Low blood pressure (average 70/40 Went to ER Sunday for hydration and low BP Wednesday 1/20/21 diarrhea x 25 episodes while at work, Sent home at 3:30pm. Body aches, chills, sever abdominal pain. | |
| COVID19 VACCINE (COVID19) | Patient died 4 days after immunization. Probably unrelated to immunization, as patient has been in poor health and was receiving hospice services. I have no details related to his illness or symptoms. Daughter is the HIPAA/emergency contact and will have all the information needed. | |
| COVID19 VACCINE (COVID19) | Sudden Sensorineural Hearing Loss in left ear. Symptoms began Friday evening Jan. 8, 2021. Sounded like muffled sound in my ear, water running, ringing. Then on Saturday Jan.9, 2021 my left ear felt like it had to pop and I felt my hearing was impaired. By Sunday evening Jan. 10, 2021, I could barely hear out of my left ear. I called MD immediately Monday morning, Jan. 11, 2021 and was seen that afternoon. I was examined and had a hearing test. I was diagnosed with SSHL and started treatment of a series of steroid injections directly into my eardrum to save my hearing immediately. I have had 2 injections and hearing test since then. The doctors feel this was a side effect of the COVID vaccine due to my compromised immune system, but not an allergic reaction, but a side effect. I had the same condition about 15 years ago from a virus. | |
| COVID19 VACCINE (COVID19) | Pt passed away the day after the vaccine was given. |
| COVID19 VACCINE (COVID19) | 12 hours after vaccination began experiencing fever, chills, body aches, slight head ache – lasted around 12 hours Had slight pain above eye prior to getting vaccination Saw PCP on 01/08/2021 due to eye pain – had CT scan for possible aneurysm, found 2 spots on brain, thought patient had shingles On 01/10/2021 shingles rash appeared | |
|---|---|---|
| COVID19 VACCINE (COVID19) | I was having episodes of dyspnea and non productive cough starting from 1/1/2021. On 1/13/2021 I experienced severe dyspnea and had loss of consciousness for 5 seconds and was found down. I was rushed to the hospital and diagnosed with multiple pulmonary embolus (about 9) which was treated with direct TPA via catheterization. I then recovered in the ICU and transitioned to oral anticoagulation and discharged home on 1/15/2021. | |
| COVID19 VACCINE (COVID19) | Anaphylaxis Allergic reaction COVID-19 vaccine: dizziness, vomiting and shortness of breath. Received vaccine and about 5/10 minutes later developed symptoms of chest tightness shortness of breath wheezing. Arrived to ED at 1156 and discharged at 1507. Given epi IM Solu-Medrol, Pepcid, Benadryl, albuterol. | |
| COVID19 VACCINE (COVID19) | 1/4/21- Patient stated she had tenderness on the back of her left lower leg with redness then 1/8/21 started to have shortness of breath and made a doctor’s appointment for 1/13/21. Seen by provider on 1/13/21 and was sent to ED and admitted to the hospital [ICU] with NSTEMI, acute deep, occlusive venous thrombosis left femoral vein and saddle embolus of pulmonary artery. Transferred to another acute care hospital for removal of thrombosis. Patient started on Eliqus and no intervention for removal of the thrombosis. | |
| COVID19 VACCINE (COVID19) | Patient received her first dose of the Moderna COVID-19 Vaccination on Saturday January 16th 2021 at approximately 12pm. She completed all necessary screening forms and was deemed to be at low risk for serious allergic reactions. She tolerated the vaccination well, and no complications or immediate adverse events occurred. She was observed for a full 15 mins per CDPHE/CDC guidelines and left the Clinic in stable condition after her observation period was complete. On the morning of Tuesday, January 19th, 2021, the patient was found unconscious and unresponsive by her husband. She was transferred by Ambulance to Hospital shortly thereafter. She was diagnosed with a brain bleed that was determined to be inoperable. She was transferred to other Hospital for higher level care. She was seen by neurosurgery and diagnosed with a ruptured aneurysm. She was treated in the ICU for 24 hours, at which point her team determined that the severity of her brain bleed would not respond to treatment. Supportive cares were withdrawn on Wednesday Jan 20th, and she passed away shortly thereafter. | |
| COVID19 VACCINE (COVID19) | Resident has increase weakness and lethargy with abnormal labs. He was transferred to the ER. He was admitted to the hospital and treated for worsening AKI and hypotension. | |
| COVID19 VACCINE (COVID19) | Appendicitis, presenting as periumbilical tenderness at onset (26 hrs after vaccine admin) migrating to RLQ approx 20hrs later (46hrs after vaccine admin) accompanied by fever, chills, sweats, and nausea. Presented to ER that evening and CT confirmed appendicitis (52hrs after vaccine admin). Surgery following day laparoscopic appendectomy (69hrs after vaccine admin). Recovery and clinical improvement over next 8hrs (77hrs after vaccine admin). Discharged following day (96hrs after vaccine admin) | |
| COVID19 VACCINE (COVID19) | 3 days post = tremors; 4 days post= pneumonia; 6 days post= hospitalized | |
| COVID19 VACCINE (COVID19) | Patient unresponsive post vaccine. Taken to hospital. Please contact facility for full Report. | |
| COVID19 VACCINE (COVID19) | Per Nursing Staff- patient died within 24 hours of receiving the vaccine. patient has hospice. Please contact director of nursing for more details. | |
| COVID19 VACCINE (COVID19) | Started itching within (left arm) 15 minutes. THey said I was fine and to go back to work. About an hour later, I started breaking out in hives and whole body itching. I went back in and they gave me to full strength Benadryl and it was not helping and my BP was 190/140 (stroke level) and they tried to bring that down. About 10:15 my face was starting to swell and I was short of breath and 10:30 they took me to ER – and gave me Cortisol shot. And IV fluids. And I was in ER for two hours. They wrote me a prescription for six days for 2 prednisone for every day for one week. The PA saw me at the ER and he prescribed. I went home but couldn’t drive home because I couldn’t see straight so got a ride home. They tested my O2 levels before they left me. Oxygen was 96. My blood pressure was down to 140/95 – so it was down but still elevated. I still had facial swelling for 3 days. But after three or four days it resolved the face swelling. Had a weakness from the shot and still itching but nothing like it was that day still after the four days. Dr. told me I couldn’t get second dose. It was an anaphalactic reaction. Dr – prescribed me an EpiPen in case I have another bad reaction to anything. | |
| COVID19 VACCINE (COVID19) | per staff at facility patient died 24 hours post vaccination. Please contact Director of Nursing for further details. | |
| COVID19 VACCINE (COVID19) | Swelling all over her body, ear popping all the time, hands and feet are numb, torso swollen and numb, face swollen and red. Taking steroids for four days. Went to hospital. Then went to other facility. | |
| COVID19 VACCINE (COVID19) | short of breath Narrative: patient complained of shortness of breath prior to getting covid vaccine, patient and wife stating it was his norm. After vaccine he complained of increasing shortness of breath, and hypoxic with bluish nail beds, lips, and greyish in color. Applied O2 via mask, and nail beds, lips, and facial color returned, sent patient to local ER for treatment and evaluation. | |
| COVID19 VACCINE (COVID19) | “Narrative: Patient seen in ED 1-17-21 with c/c of “”bloated with epigastric pain””. Patient with complicated medical history including stage 1B pancreatic cancer (was currently on chemotherapy mFOLFIRINOX), and a leadless permanent pacemaker implantation on 1-11-21 for long episodes of SR with complete heart block following symptoms of syncope (other cardiac history: CAD s/p CABG 2009, PAF, and HTN). Regarding ER visit for epigastric pain, nothing notable was found on workup and patient was to discharge home to rest. There were available doses of COVID-19 Vaccine following a vaccine clinic that same day, and patient was offered and agreed to a dose of vaccine. Patient was monitored for 15 minutes post vaccine with no notable issues. The following day, Monday 1-18-21, patient’s caregiver called facility at 22:30 to report he had a fever of 102.8 degrees and that he had been “”feeling kind of bad all day””. Patient was advise to seek urgent medical care and reported back to ED on 1-19-21 at 00:55. Patient wasd admitted for SIRS (tachycardia and febrile) — patient also reported diffuse myalgia. WBC WNL, CXR unremarkable for infection, UA neg for bacteria, LFTs WNL, blood cultures negative. Procalcitonin elevated at 17.8 — suggesting inflammatory response. Patient initially reported feeling better on the morning of 1-19-21, but around 13:00 began rapidly declining (confusion, unable to walk) and started experiencing EKG changes (9 beats of SVT). Patient then coded and resuscitation was attempted for approximately 30 minutes. Patient did not survive the code. Coroner has been notified and family is considering autopsy at time of this report.” | |
| COVID19 VACCINE (COVID19) | Vaccine was administered very high, presumably in the joint space, rather than the deltoid. Patient experienced intense pain in the entire shoulder area for 24 hours following administration. After initial 24 hours, pain remained more localized in the joint itself. Pain is intensified when moving out of the neutral position. | |
| COVID19 VACCINE (COVID19) | significant facial/lip angioedema first noted ~20 hours post vaccination, leading to intubation in ED due to concern for airway protection. extubated and discharged in 2 days | |
| COVID19 VACCINE (COVID19) | Admitted in Hospital for Anaphylaxis. | |
| COVID19 VACCINE (COVID19) | Noticed small area of burning sensation at right side of wrist and the vein there was very enlarged and sticking out. | |
| COVID19 VACCINE (COVID19) | Began itching and wheezing approximately 5 minutes after the injection. Gave first epi dose. Throat started tightening, and nausea presented. Gave second epi 5 min after the first. Gave third epi 5 min after the second. EMS arrived, gave 4th epi in ambulance. ER treated with breathing treatment, IV steroids, IV Benadryl, IV Pepcid and IV zofran. Was observed for 6.5 hours. | |
| COVID19 VACCINE (COVID19) | Patient came to ED at 1600 with right upper lip swelling and finger swelling after getting covid vaccine earlier. Angioedema of lips, initial encounter; History of allergic reaction; Lip swelling; Vaccination side effects, initial encounter. Pt has history of rheumatoid arthritis. Was treated & discharged home on 1/12/21 | |
| COVID19 VACCINE (COVID19) | Slurred speech started morning of 1/8 and patient went to ED after dialysis appointment. Admitted for TIA (transient ischemic attack). Discharged home on 1/10 with follow up appts with Neurology. | |
| COVID19 VACCINE (COVID19) | Presented to ED 1/12 with primary complaint of Fatigue starting that AM. Being treated for Stage IV Sacral Decubitus Ulcer w/ possible osteomyelitis, Still admitted |
| COVID19 VACCINE (COVID19) | Pt reported difficulty in swallowing and wife noticed left-sided facial droop morning of 1/10. Patient admitted for concerns of TIA. Symptoms resolved prior to hospitalization. Patient had MRI brain without contrast of the find evidence of acute infarct. Neurology recommended treatment patient has TIA and having dual anti-platelet therapy for 21 days followed by monotherapy of Plavix for stroke prevention. Patient was stable discharge to home 1/12/21 | |
|---|---|---|
| COVID19 VACCINE (COVID19) | Patient was receiving dialysis and had low grade fevers on the morning on 1/15/2021. Patient was sent to the hospital’s emergency room and was found to have a temperature of 103. The patient also had mental status changes. It is unsure what caused the mental status changes and fevers. | |
| COVID19 VACCINE (COVID19) | Pt presented to ED with Left facial numbness and concern for stroke. Observed over night. MRI brain negative for acute process. Stable at baseline neuro status 1/10/21, discharged home. | |
| COVID19 VACCINE (COVID19) | Pt Rec’d Covid vaccine and injection in Lt eye for macular degeneration. Monday 1/11 slurring speech/jumbled words since dinner, went to bed, wife states improved from last night but still difficult clearly communicating. Also reports difficulty writing. Came to ED and admitted for stroke evaluation. Stable for discharge home 1/13 with neurology follow up visits. | |
| COVID19 VACCINE (COVID19) | Pt presented to ED 1/12 complaining of shortness of breath and nonproductive cough which onset approximately 8 days ago. Tested positive for COVID. Remains admitted for management of COVID. | |
| COVID19 VACCINE (COVID19) | Stated since Xmas he has not feeling well after a family gathering. His wife in hospital for Covid-19 pneumonia. He reports for about 1 week, his SOB worsen, not eating well at all for the past 3 days. Which prompt him to visit the ED. Admitted to Hopital for Dehydration; Dyspnea; Pneumonia due to COVID-19 virus; COVID+ 1/10/21; still admitted | |
| COVID19 VACCINE (COVID19) | expired before receiving the second dose; This is a spontaneous report from a contactable nurse. This nurse reported similar death events for 8 patients. This report is for 1st of 8 patient. A patient of unspecified age and gender received first dose of bnt162b2 (PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration on 22Dec2020 at single dose for covid-19 immunisation. The patient medical history was and concomitant medications were not reported. The patient expired before receiving the second dose on an unspecified date. It was not reported if an autopsy was performed. Information about Lot/Batch number is requested.; Sender’s Comments: Current information is very limited for full assessment. The patient died following the vaccine use; further information such as patient demographics, complete medical history, concomitant medications, concurrent illness and event term details especially death cause and autopsy results are needed for meaningful evaluation. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate.,Linked Report(s) : US-PFIZER INC-2021034597 same drug, reporter and event but different patient;US-PFIZER INC-2021034598 same drug, reporter and event but different patient;US-PFIZER INC-2021034599 same drug, reporter and event but different patient;US-PFIZER INC-2021034600 same drug, reporter and event but different patient;US-PFIZER INC-2021034601 same drug, reporter and event but different patient;US-PFIZER INC-2021034603 same drug, reporter and event but different patient;US-PFIZER INC-2021034596 same drug, reporter and event but different patient.; Reported Cause(s) of Death: expired before receiving the second dose | |
| COVID19 VACCINE (COVID19) | expired before receiving the second dose; This is a spontaneous report from a contactable nurse. This nurse reported similar events for 8 patients. This report is for 2nd of 8 patients. A patient of unspecified age and gender received the first dose of bnt162b2 (PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration on 22Dec2020 at single dose for covid-19 immunisation. The patient medical history and concomitant medications were not reported. The patient expired before receiving the second dose on an unspecified date. It was not reported if an autopsy was performed. Information about Lot/Batch number is requested.; Sender’s Comments: Current information is very limited for full assessment. The patient died following the vaccine use; further information such as patient demographics, complete medical history, concomitant medications, concurrent illness and event term details especially death cause and autopsy results are needed for meaningful evaluation. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate.; Reported Cause(s) of Death: expired before receiving the second dose | |
| COVID19 VACCINE (COVID19) | expired before receiving the second dose; This is a spontaneous report from a contactable nurse. This nurse reported similar events for 8 patients. This report is for 3rd of 8 patients. A patient of unspecified age and gender received the first dose of bnt162b2 (PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration on 22Dec2020 at single dose for covid-19 immunisation. The patient medical history and concomitant medications were not reported. The patient expired before receiving the second dose on an unspecified date. It was not reported if an autopsy was performed. Information about Lot/Batch number is requested.; Sender’s Comments: Based on the reasonable temporal association, the Company cannot completely exclude the possible causality between the reported death and the administration of COVID 19 vaccine, bnt162b2. However, more information on the patient’s underlying medical condition, concomitant medications, patient’s age group, clinical course and relevant lab tests would be helpful for the Company to make a more meaningful causality assessment. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to RA, IEC, as appropriate.; Reported Cause(s) of Death: expired before receiving the second dose | |
| COVID19 VACCINE (COVID19) | expired before receiving the second dose; This is a spontaneous report from a contactable nurse. This nurse reported similar events for 8 patients. This report is for 4th of 8 patient. A patient of unspecified age and gender received the first dose bnt162b2 (PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration on 22Dec2020 at single dose for covid-19 immunization. The patient’s medical history and concomitant medications were not reported. The patient expired before receiving the second dose on an unspecified date. The patient died on an unspecified date. It was not reported if an autopsy was performed. Information about Lot/Batch number has been requested.; Sender’s Comments: Current information is very limited for full assessment. The patient died following the vaccine use; further information such as patient demographics, complete medical history, concomitant medications, concurrent illness and event term details especially death cause and autopsy results are needed for meaningful evaluation. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate.,Linked Report(s) : US-PFIZER INC-2021034595 same drug, reporter and event but different patient; Reported Cause(s) of Death: expired before receiving the second dose | |
| COVID19 VACCINE (COVID19) | 7 residents expired before receiving the second dose; This is a spontaneous report from a contactable nurse. This nurse reported similar events for 8 patients. This report is for 6th of 8 patients. A patient of unspecified age and gender received first dose bnt162b2 (PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration on an unspecified date at SINGLE DOSE for covid-19 immunisation. The patient’s medical history and concomitant medications were not reported. The patient expired before receiving the second dose on an unspecified date. It was not reported if an autopsy was performed. Information on the lot/batch number has been requested.; Sender’s Comments: The event death is assessed as related to BNT162b2 vaccine and documented as such in the global safety database until sufficient information is available to allow an unrelated causality assessment. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to regulatory authorities, Ethics Committees, and Investigators, as appropriate.,Linked Report(s) : US-PFIZER INC-2021034595 same drug, reporter and event but different patient; Reported Cause(s) of Death: 7 residents expired before receiving the second dose | |
| COVID19 VACCINE (COVID19) | expired before receiving the second dose; This is a spontaneous report from a contactable nurse. This nurse reported similar death events for 8 patients. This report is for 7th of 8 patient. A patient of unspecified age and gender received first dose of bnt162b2 (PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration on 22Dec2020 at single dose for covid-19 immunisation. The patient medical history was and concomitant medications were not reported. The patient expired before receiving the second dose on an unspecified date. It was not reported if an autopsy was performed. Information about Lot/Batch number is requested.; Sender’s Comments: Current information is very limited for full assessment. The patient died following the vaccine use; further information such as patient demographics, complete medical history, concomitant medications, concurrent illness and event term details especially death cause and autopsy results are needed for meaningful evaluation. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to Regulatory Authorities, Ethics Committees and Investigators, as appropriate.,Linked Report(s) : US-PFIZER INC-2021034595 same drug, reporter and event but different patient; Reported Cause(s) of Death: expired before receiving the second dose | |
| COVID19 VACCINE (COVID19) | platelets dropped so low/thrombocytopenia; Hemorrhagic stroke/brain hemorrhage; This is a spontaneous report from a contactable nurse. A 56-year-old male patient received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE) via an unspecified route of administration on 18Dec2020 at single dose for covid-19 immunisation. Medical history and concomitant medications were unknown. The reporter read about the doctor that died that developed thrombocytopenia after taking the vaccine, stated it was in the news yesterday. The patient received the Pfizer Covid vaccine on 18Dec2020, and he died 16 days later from a brain hemorrhage. Autopsy stated that said he had a hemorrhagic stroke on 03Jan2021. His platelets dropped so low that he had specialists that tried to get his platelet count back up again and they could not get his platelets back up again and he ended up having the hemorrhagic stroke. The reporter already had thrombocytopenia and she was debating what she should do about getting vaccine. Outcome of the events was fatal. Information on the lot/batch number has been requested.; Sender’s Comments: Very limited information is currently available. Lacking patient’s underlying medical conditions, clinical course, relevant lab data, the Company cannot make a meaningful causality assessment. The reported hemorrhagic stroke following low platelet count are managed as related to the suspect, BNT162B2, for reporting purpose only. The impact of this report on the benefit/risk profile of the Pfizer product is evaluated as part of Pfizer procedures for safety evaluation, including the review and analysis of aggregate data for adverse events. Any safety concern identified as part of this review, as well as any appropriate action in response, will be promptly notified to RA, IEC, as appropriate.; Reported Cause(s) of Death: Hemorrhagic stroke/brain hemorrhage; platelets dropped so low/thrombocytopenia | |
| COVID19 VACCINE (COVID19) | “died; tested positive for COVID; tested positive for COVID; This is a spontaneous report from a contactable consumer from a Pfizer-sponsored program, Pfizer First Connect. A 97-year-old male patient received the first dose of the bnt162b2 (PFIZER-BIONTECH COVID-19 MRNA VACCINE), via an unspecified route of administration on 30Dec2020 at 97-years-old at a single dose for COVID-19 immunization; administered by the nursing home. Medical history included glaucoma from an unknown date and unknown if ongoing. Concomitant medications included: “”used a sav for skin tears””, and “”eye drops for glaucoma”” from an unknown date to an unknown date. On 07Jan2021, the patient experienced: tested positive for COVID (medically significant). The patient died (death, medically significant) on 17Jan2021. The clinical course was reported as follows: The reporter stated that in regard to the patient’s height and weight: “”was probably getting down to about five foot eight. Shrinking.”” The reporter stated that If she remembered correctly, they were trying to maintain the patient’s weight 135 to 136 pounds. The reporter stated that her father was in a nursing home. The patient received his first dose of the COVID vaccine on 30Dec2020. The patient died on 17Jan2021. The reporter stated that she “”wanted Pfizer to know that the little old people in the nursing might not be strong enough for the vaccine.”” The reporter stated that she was “”not calling to complaining.”” The reporter stated that there was nothing wrong with her dad. He was elderly with no health issues. “”He was literally on no medications. The only reason he was in the nursing home was because he was afraid to walk.”” The reporter stated that she received a call about giving the patient the vaccine and she said yes because she wanted him to have the vaccine. One week after the vaccine, the patient tested positive for COVID “”like all the other people”” (no further details provided). The reporter stated that her dad had no symptoms of COVID. The director of nursing said the patient was doing so well. The patient ate his lunch, he laid down for nap, and at 14:30 he was gone. The patient “”went peacefully in his sleep.”” The reporter then again stated that the patient literally had nothing wrong with him. “”They were shocked. They fed him and he took a nap. He was sleeping, but it was eternally.”” The reporter stated that, “”it might not have been the Pfizer vaccine, maybe his heart wore out.”” In regard to an autopsy: the reporter stated that they would get it done if needed. The patient underwent lab tests and procedures which included COVID-19 virus test: positive on 07Jan2021. History of all previous immunization with the Pfizer vaccine considered as suspect: none. It was unknown if there were additional vaccines administered on the same date of the Pfizer suspect, but the reporter doubted it. There were no prior vaccinations within 4 weeks. There were no adverse events following the prior vaccinations. The clinical outcome of the event, died, was fatal. The clinical outcome of the event, tested positive for COVID, was unknown. The patient died on 17Jan2021 due to an unknown cause of death. An autopsy was not performed. The batch/lot numbers for the vaccine, PFIZER-BIONTECH COVID-19 MRNA VACCINE, were not provided and will be requested during follow up.; Reported Cause(s) of Death: died” | |
| COVID19 VACCINE (COVID19) | At approximately 930am I arrived at Memory Care. I met with the director of the facility and she directed me to where my team would be setting up. My team consisted of (technician), (nurse) and I. As we were setting up, the director asked how she can help. I explained to her that we would need a designated area for patients to be monitored after vaccination for 15 minutes and maybe even longer . I also explained that we would need one of her staff monitoring while we vaccinate. She agreed, and proceeded to designate her staff and the cafeteria area, facing the vaccination station,the monitoring station. Throughout the day, nurse and I were both vaccinating,while the staff of the facility would monitor the vaccinated patients. I would also stop occasionally to mix the vaccine and check the temperature of the aero safe. At approximately 12:50pm, the director rushed in and stated that a patient is not responding, and that she had been vaccinated. At that point, I grabbed epipens and a thermometer and I also instructed nurse to grab an Epipen and come with me. We followed the director to pt’s room. Once we got to the room, the patient was in bed and there were 4 staff members standing bedside and one of them turned and stated the patient has passed. At that point I asked the staff how long ago did the patient get the vaccine, they stated about 30 minutes ago. They also stated that the patient was a hospice patient and that the patient had declined, and was rapidly detiorating and had not eaten or drank anything all day . They also stated that the patient had been monitored for 15 minutes post vaccination. I then left the room and grabbed the patients COVID Vaccine intake consent form. I looked at the answered questionaire and all the responses were circled NO. Patient had a temp of 96.5 at the time of vaccination.The vaccine administration information for Immunizer Section was filled out by Nurse. I then proceeded to ask the director once again if there were staff that was monitoring her for 15 minutes, the director stated they had staff monitoring her. She also stated the Hospice nurse has to announce her death, so they waited for the Hospice Nurse to come. I then called Corporate and explained the situation. After speaking to corporate, I also asked nurse, if she remembered the patient. She stated that she did and at the time of the vaccination the patient was not alert, there were two staff members with the patient. She was non oriented and she kept closing her eyes. At that point, Nurse stated that she asked the two staff members with her if this is how she usually is and if its ok to vaccinate her. Both Staff members stated that it its ok,this is how she is. The Nurse then proceeded to vaccinate. At approximately 3:10pm, as I was leaving I spoke to the director, and one of her Staff members. Staff that the patient has actually not eaten/ or drank anything for the past several days, including today(01/18/21). Staff also stated that on Friday, Jan 15th,2021, they had informed the family that the patient was rapidly detiorating. Staff also stated that the family knowingly gave the consent to vaccinate her. She also stated that the hospice Nurse believes that the death was primarily caused by her detiorating state. She also stated that the hospice Nurse informed that the death was not due to the Vaccine. Per Lead Pharmacist at the clinic. | |
| COVID19 VACCINE (COVID19) | Extreme Fatigue |
| COVID19 VACCINE (COVID19) | Patient developed 104.4 temp approximately 48 hours after being given the vaccine. I treated him with antibiotics, IV fluids, cooling methods. CXR does show a new right perihilar infiltrate. However, his fever came down within the next 24-48 hours. Unfortunately, he suffered a cardiac arrest on 1/21/21 in the early morning and expired. | |
|---|---|---|
| COVID19 VACCINE (COVID19) | Resident returned to the memory support unit at 1500. Resident was than toileted and transferred in to bed per his request. At 1515 resident was observed face down beside bed, resident sustained a 1inX1in eccyhmotic/hematoma to the forehead. Neuro Checks with in normal limes Vital signs: 100/52, 100, 97.2, 28. Resident sent to ED for further medical evaluation via EMS. | |
| COVID19 VACCINE (COVID19) | possibly got it at clinic, possibly who administered shot. Pts. daughter said the pts boyfriend denied any symptoms the whole day but that in the middle of the night the pt passed away. | |
| COVID19 VACCINE (COVID19) | This is a 94-year-old male who is brought in by ambulance after being found on the floor with unknown downtime. He was in asystole upon EMS arrival. He remains in asystole. No advanced airway is in place. The patient is getting compressions from Lucas device upon arrival. It was reported that he was last talked to by family at 2 PM. The patient got his SARS-CoV-2 vaccination this morning. The patient is evaluated emergently. CPR was ongoing with 3 rounds of epinephrine given. The patient remains in asystole. He has rigor mortis. The patient’s pupils are fixed and dilated. The patient has compressions paused and ultrasound is used to evaluate for cardiac activity. None is detected. The patient has no electrical activity on monitor. The patient’s time of death is 2113. | |
| COVID19 VACCINE (COVID19) | approximately 3 hours prior to expiring the patient was experiencing forceful emesis. later was found to have expired, patient was comfort care only. | |
| COVID19 VACCINE (COVID19) | Theápatientáreceivedáhisávaccineáináthe.ámorningáofá1/20/2021,áwhileágettingáintoácarátoágoáseeáhisápulmonologist,áaboutá2áhoursáafter,ácollapsed,áunresponsiveáwitháasystolicácardiacáarrest.ááNoásymptomsáprioráotheráthanáchronicádyspnea.ááNoáallergicátypeásymptomsáreportedábyáfamily.ááAsystoleáwitháEMS,ánoáresponseátoáACLS,ápresentedátoáED,áDOA. | |
| COVID19 VACCINE (COVID19) | 1/13/2021 12:00 PM: Patient received COVID-19 Vaccine. 1/14/2021 21:00: Nurse performed routine rounds and the patient appeared okay. 1/14/2021 22:00: CNA discovered patient unresponsive in bed, began CPR, and called 911. 1/14/2021 23:08: Pronounced deceased. | |
| COVID19 VACCINE (COVID19) | Narrative: | |
| COVID19 VACCINE (COVID19) | “Narrative: Was pt previously covid positive?- Yes. Initial- 10/27/2020, 11/29/2020, 12/22/2020 Are there any predisposing factors for patient experiencing adverse drug event?- Yes, patient had multiple co-morbidities including GI bleed, hepatitis congestion due to cardiac issues, treatment for PE, NSTEMI, or antibiotics for PNA, also on concurrent medications APAP, Atorvastatin, Mirtazapine and Duloxetine. Pt with 2 doses of covid-19 vaccine, second one on 01/08/2021, 2 days pre-death Any occurrence of an ADR at time of administration? Did not specify injection site issues, per RN admin note- Vaccine “”administered without complications.”” Did patient recover from event? Not s/p dose on 01/08/2021. First dose given on 12/21/2021, LFTS increased ~01/01/2021, peaked on 01/03/2021 and were decreasing on 01/07/2021 Was there an ADR between observation period and date of death? No Did patient recover from event? No (01/08/2021 event, died 01/10/2021) Was patient hospitalized prior to vaccination? Yes, in between inpatient and nursing home Was patient hospitalized prior to death–was hospitalization attributable to ADE? Yes re-admitted to inpatient on 12/31/2020. GI bleed Is there an alternative cause of death? Yes, as noted above. Quite a complicated case with many comorbidities/concurrent medications as noted above. Primary Diagnosis: Upper GI Bleed in the death note from 01/10/2021″ | |
| COVID19 VACCINE (COVID19) | tired; legs felt heavy; stopped breathing; This is a spontaneous report from a Pfizer-sponsored program a non-contactable consumer. A 93-year-old male patient received bnt162b2 (PFIZER-BIONTECH COVID-19 VACCINE), via an unspecified route of administration on 04Jan2021 11:00 at single dose for covid-19 immunisation. The patient medical history and concomitant medications were not reported. Patient received vaccine around 11:00 a.m. About two hours later, he said he was tired and couldn’t continue with the physical therapy he was doing. He was taken back to his room, where he said his legs felt heavy. Soon after, he stopped breathing. A nurse declared a do-not-resuscitate order. The patient died on 04Jan2021. It was not reported if an autopsy was performed. Outcome of stopped breathing was fatal. Outcome of tired and legs felt heavy was unknown. No follow-up attempts are possible; information about lot/batch number cannot be obtained.; Reported Cause(s) of Death: stopped breathing | |
| COVID19 VACCINE (COVID19) | died; This is a spontaneous report from a Pfizer-sponsored program. A contactable consumer reported that an 83-year-old female patient (reporter mother) received BNT162B2 (PFIZER-BIONTECH COVID-19 VACCINE, Solution for injection), via an unspecified route of administration on an unspecified date at single dose for covid-19 immunization. Medical history included hospice care and dementia. The patient’s concomitant medications were not reported. The patient died one day after getting vaccine. She was reportedly in good health the day before receiving vaccine. She was on hospice, frail, but in good condition and checked by a hospice nurse the day before which she reported her in good health considering. She was with dementia but stable in her health. The reporter read investigating 23 deaths of people receiving vaccine in similar conditions. The patient died on an unspecified date. It was not reported if an autopsy was performed.; Reported Cause(s) of Death: died | |
| COVID19 VACCINE (COVID19) | “Called to schedule second vaccine and daughter reports that he died on01/19/2021 with “”COVID””” |
| COVID19 VACCINE (COVID19) | “Patient’s wife called this morning stating that her husband has passed away last night. After receiving first dose of Pfizer COVID-19 vaccine at around 0830, patient remained in the Immunizations Department for the 15-minute monitoring period. Per wife, patient’s only complaint was pain at the injection site. At 1300, wife states that patient complaint of dizziness which “”dissipated after a few minutes”” followed by a headache which “”dissipated after a few minutes”” as well. Then patient complained of nausea, no vomiting and “”couldn’t relax.”” Per wife, from around 1400/1500, patient stayed on his recliner while still having a conversation with her–“”he didn’t get up to eat.”” Last conversation they had was around 2000/2100. Per wife, at around 2100/2200, patient was quiet and when she checked on him, “”he wasn’t responding anymore.”” Wife then called 911, “”but they couldn’t revive him.””” | |
|---|---|---|
| COVID19 VACCINE (COVID19) | Admitted to hospital after vaccination with Acute hypoxemic respiratory failure, Septic shock; Aneurysm of arteriovenous dialysis fistula; expired 1/16/2021 | |
| COVID19 VACCINE (COVID19) | We do not believe that the patient’s death was an adverse event from the vaccine. Patient received COVID vaccine from Pfizer Dose #1 12/19/2020 (lot # EK5730) and Dose #2 1/7/2021 (lot # EL1284). No side effects or adverse events noted; lived in 24/7 care facility and monitored twice daily for reaction. Patient died 1/10/2021 from chronic respiratory failure and congestive heart failure after recent aspiration pneumonia requiring hospitalization. Death was anticipated and not sudden. We were told to report his death to VAERS even though his death was anticipated and not related to his vaccination. | |
| COVID19 VACCINE (COVID19) | Patient deceased | |
| COVID19 VACCINE (COVID19) | Patient did not have any adverse reaction to the COVID vaccine, but we were asked by our health dept to submit a VAERS report since the patient died between his first and second dose. Received Pfizer Dose #1 12/17/2020. No side effects or adverse events noted; lived in 24/7 care facility and monitored twice daily for reaction. Date of death 12/23/2020 from aspiration pneumonia complicated by end-stage heart failure and ischemic cardiomyopathy. Death was anticipated and not sudden. | |
| COVID19 VACCINE (COVID19) | patient expired 1/15/2021; had been treated as outpatient for pneumonia, likely COVID-19 but no positive test result in December 2020. PMH diabetes | |
| COVID19 VACCINE (COVID19) | Admitted 1/14/21: Patient is an elderly 93-year-old female with multiple medical problems including chronic combined CHF, P 80, diabetes mellitus, HTN, hyperlipidemia, CKD stage 3, has been complaining of generalized weakness, fatigue, decreased appetite for the past few days. She had an outpatient COVID-19 vaccine earlier today. Within 2 hr of admitting the patient to the hospital, condition clinically deteriorated. Patient elected to be DNR/DNI while in the ED. Patient was pronounced dead at 10:30 p.m. earlier today. Preliminary cause of death: Hypoglycemia induced lactic acidosis. | |
| COVID19 VACCINE (COVID19) | Pt received second dose of COVID vaccine on 01/20/2021 at 1430. At 1600 Pt developed a wet productive cough with coarse crackles. Pt ate dinner at 5 pm cough persisted. At 18:30 the nurse went to Pt’s room to give him his medications. Pt still had a cough, denied shortness of breath. Pt was in a good mood and joking with staff. Pt asked to be shaved. At 19:45 Pt was sitting in the lounge and a CNA noticed that Pt was pale/white in color and clammy. 02 Sat was 85%. Respirations were labored. Pt was placed on 4 L of 02. Increased to 5 L via face mask and 02 sat was 89-90%. Ambulance was called at unknown time. Pt arrived at Medical Center at 2120 and was pronounced dead at 2127. | |
| COVID19 VACCINE (COVID19) | On Saturday, 1/16/2021, Patient went to the grocery store. Upon her return, she indicated she was experiencing N/V and some throat swelling. Patient subsequently collapsed and expired before she could be brought to an emergency room. During investigation by Coroners Office, it has been reported that Patient may have gotten some takeout food while she was out. Labs are pending and the Coroners investigation is ongoing. Spouse believes that her death was caused by the vaccine. | |
| COVID19 VACCINE (COVID19) | No immediate reaction. Patient-reported deceased four days later on Jan. 19, 2021. As of this date cause of death is unknown to our clinic. | |
| COVID19 VACCINE (COVID19) | unknown. Event occurred after leaving vaccination site | |
| COVID19 VACCINE (COVID19) | presented to ED 1/9/21 with abdominal pain, progressive worsening weakness and fatigue and new onset A fib with RVR likely due to hypertensive urgency . Patient progressed clinically with severe hypoxia and transferred to ICU and started on BiPAP; progressive decline with decreased urinary output with uremia likely secondary to sepsis. Concern with patient worsening clinical decline, palliative care had been consulted on end of life care. Patient expired 1/17/21 | |
| COVID19 VACCINE (COVID19) | Narrative: | |
| COVID19 VACCINE (COVID19) | Fever, Tachypnea, HYPERtension, Tachycardia & HYPERglycemia Narrative: Was inpatient overnight in a telemetry until. DC with diagnosis of DM and CHF | |
| COVID19 VACCINE (COVID19) | 1/11 asymptomatic COVID Positive test Narrative: | |
| COVID19 VACCINE (COVID19) | Narrative: Symptoms: & Cardiac Arrest; Death Treatment: EPINEPHRINE | |
| COVID19 VACCINE (COVID19) | shaking, altered consciousness Narrative: One day after pt received his first covid vaccine, pt experienced upper extremity shaking leading to ED visit and subsequent hospitalization with concern for seizure. Examination and labs were not consistent with seizure. He had features of lewy body disease and parkinsonism. Labs were significant for leukocytosis, but pt had no other signs/symptoms of infection or findings to indicate a source of infection. Pt referred to Neurology. | |
| COVID19 VACCINE (COVID19) | Patient diagnosed with COVID on January 9, 2021 after being exposed to family member that was under quarantine in the same household. Admitted to the hospital and was discharged on January 14, 2021 with home hospice. Patient passed away on January 18, 2021 | |
| COVID19 VACCINE (COVID19) | Patient passed away on 01/18/2021 | |
| COVID19 VACCINE (COVID19) | Patient died unexpectedly 5 days after receiving vaccine (1/10/2021). | |
| COVID19 VACCINE (COVID19) | Patient deceased on 01/17/2021 | |
| COVID19 VACCINE (COVID19) | Death; This is a spontaneous report from four non-contactable consumers via a Pfizer-sponsored program Corporate (Pfizer) Social Media Platforms. A 78-year-old male patient received BNT162B2 (PFIZER-BIONTECH COVID-19 MRNA VACCINE), via an unspecified route of administration, on 28Dec2020 at a single dose for COVID-19 immunization. Ongoing medical history included Alzheimer’s Disease, encephalopathy, hypertension, acute kidney failure, urinary retention and recent urinary tract infection (UTI), all from an unspecified date. Concomitant medication included acetaminophen (MANUFACTURER UNKNOWN), bisacodyl (MANUFACTURER UNKNOWN), bupropion (MANUFACTURER UNKNOWN), escitalopram (MANUFACTURER UNKNOWN), hydrocodone bitartrate, paracetamol (HYDROCODONE/ACETAMINOPHEN), loperamide (MANUFACTURER UNKNOWN), ondansetron (MANUFACTURER UNKNOWN), senna alexandrina (SENNA PLUS), vitamin d3 (MANUFACTURER UNKNOWN). The patient had no known drug allergies. The patient experienced death on 30Dec2020. The vaccine was given on 28Dec2020 with no adverse events and no issues on 29Dec2020. The patient died on 30Dec2020, at approximately 2:00 AM. It was unknown if an autopsy was performed. It was unknown if the event was related to the suspect drug, the administrator marked as natural causes. No follow-up attempts are possible; information about batch/lot number cannot be obtained.; Reported Cause(s) of Death: Death | |
| COVID19 VACCINE (COVID19) | Pt died 4 days after vaccine, no known reaction to the vaccination | |
| COVID19 VACCINE (COVID19) | Death, which I believe is unrelated to vaccination | |
| COVID19 VACCINE (COVID19) | Narrative: | |
| COVID19 VACCINE (COVID19) | Death – Hospice patient with metastatic CA admitted to facility and received vaccine during stay. No adverse sequelae noted from vaccine administration, but reporting as required because pt died 7 days later. Narrative: Reporting this event because patient died 7 days after receiving vaccine in the facility where he was in hospice care for metastatic cancer. Vaccine was administered by protocol without complications. The patient had been asked and denied any prior severe reaction to this vaccine or its components and gave permission to receive it. No vaccine adverse sequelae were documented after the immunization as monitored for 15 minutes nor in facility notes for 7 days after the immunization. The patient’s death was felt to be due to underlying terminal illness. | |
| COVID19 VACCINE (COVID19) | Pt on hospice in facility for severe cardiomyopathy unable to perform interventions received vaccine without adverse sequelae died 5 days later. Reporting as required. Narrative: Reporting as required patient death 5 days after immunization with Pfizer vaccine. However, no adverse sequelae were noted to the vaccine in the 15minute observation period, nor in the days following the immunization related to the vaccine. The patient denied any prior severe reaction to this vaccine or its components, and the patient gave verbal consent to receive the vaccine. Patient had been in the facility on hospice since 11/18/20 for severe decompensated HF and newly diagnosed cardiomyopathy, unable to perform interventions, also LE ischemic wounds with very poor potential to heal due to advanced PVD. | |
| COVID19 VACCINE (COVID19) | Narrative: Temporary restriction on driving until further evaluation due to symptoms of seizures. | |
| COVID19 VACCINE (COVID19) | loss of consciousness; respiratory distress Narrative: Patient tolerated his 1st dose of the COVID-19 vaccine well, on 12/16/2020, and received his 2nd dose on 1/6/2021. Patient had some mild clinical decline the past few days prior to 2nd vaccination, with a decreased appetite and some increased fatigue per nursing report, but no significant changes. He experienced nausea on the evening of 1/6/21, which was effectively managed, but by early morning he spiked a fever of 102.9 with a sat of 86.1%. He continued to deteriorate from that point on and died 1/7/21 @13:20. Clinically, the presentation was most consistent with an aspiration pneumonia. | |
| COVID19 VACCINE (COVID19) | Death on 1-5-21 | |
| COVID19 VACCINE (COVID19) | Death 1-15-21 |